1 引 言
2 钡地球化学特征
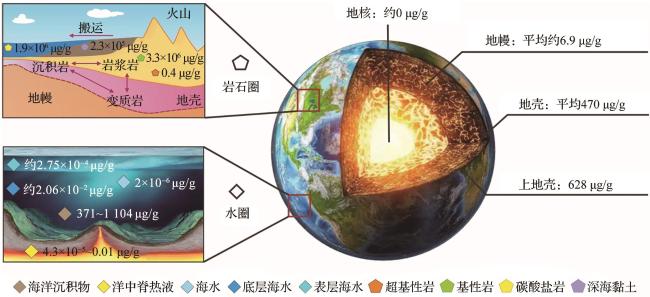
2.1 钡含量及分布
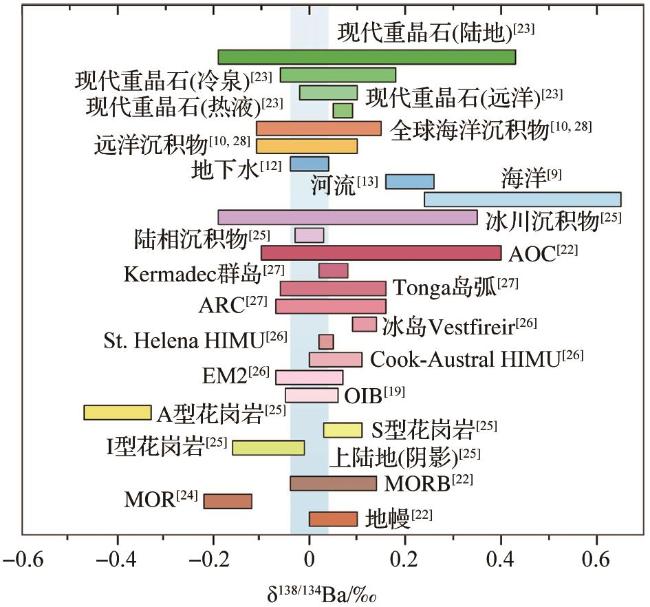
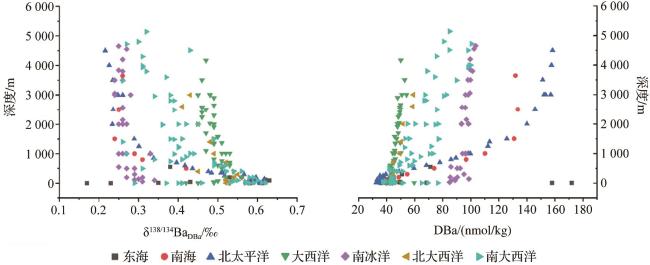
2.2 钡同位素分布
| 基本属性 | 130Ba | 132Ba | 134Ba | 135Ba | 136Ba | 137Ba | 138Ba |
|---|---|---|---|---|---|---|---|
| 原子质量 | 129.906 | 131.905 | 133.905 | 134.906 | 135.905 | 136.906 | 137.905 |
| 丰度/% | 0.106 | 0.101 | 2.417 | 6.592 | 7.854 | 11.232 | 71.698 |
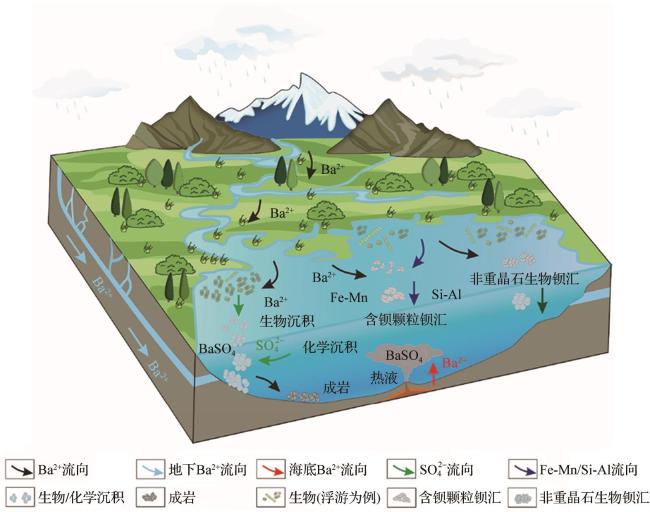
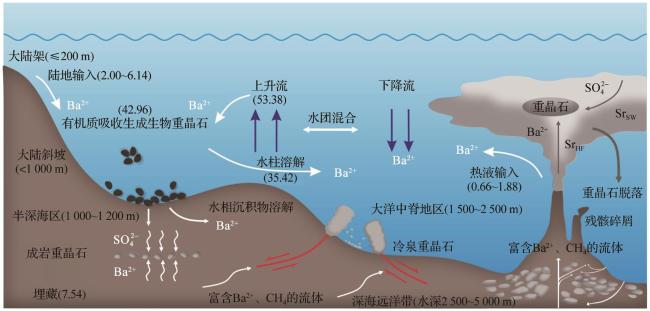
3 海洋中钡的来源
表2 钡来源及通量情况Table 2 Flux situation of barium sources |
| 钡的来源 | 通量 | 影响因素 |
|---|---|---|
| 陆源河流[1,34-36] | 全球平均2.00~6.14 nmol/(cm2·a) | 河流流域的岩石类型、土壤性质、水文条件;河口地区水体混合、吸附—解吸附、近岸陆源输入影响较大、远洋海域影响较小 |
| 三江源和祁连山海洋输出钡为9.03×10-3 Gmol/a | ||
| 黄河Baraw年通量为(0.079±0.028) Gmol/a | ||
| 河口超额钡供应通量约4.5 Gmol/a | ||
| 陆源地下水[1,12] | 0.46 nmol/(cm2·a)(约占河流通量的25%) | 排放体积流量不确定、溶质通量难约束 |
| 全球溶质通量范围为0.078~0.706 Gmol/a | ||
| 热液[1,10-11,17] | 1.00~1.39 nmol/(cm2·a) | 热液活动持续时间、海底深度、溶液温度;重晶石沉淀 |
| 2.40~3.35 Gmol/a | ||
| 2.4~6.8 Gmol/a | ||
| 生物[2,37] | 大西洋NAP站点:约2.585 nmol/(cm2·a) | 海洋生产力、生物群落结构、海域差异和环境钡浓度 |
| 赤道太平洋M站点:约5.388 nmol/(cm2·a) | ||
| 赤道太平洋H站点:约9.801 nmol/(cm2·a) |
3.1 陆源输入
3.2 热液输入
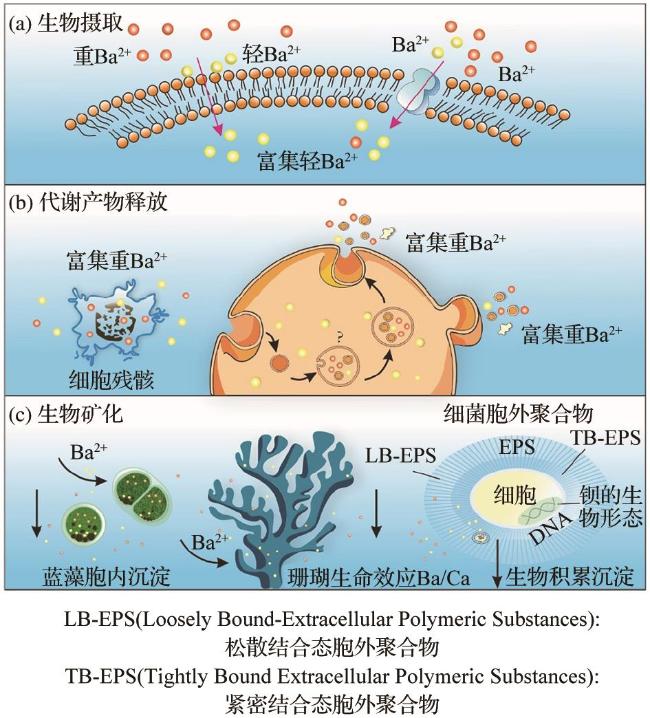
3.3 生物输入
4 海洋中钡的汇聚
4.1 重晶石钡汇
表3 重晶石成因分类与环境、鉴定特征汇总(据参考文献[1, 41]修改)Table 3 Summary of the genetic classification, environments, and identification characteristics of barite (modified after references [1, 41]) |
| 类型 | 成因分类 | 形成机理 | 形成环境 | 鉴定特征 |
|---|---|---|---|---|
| 沉积型 | 生物 | 表层水体重晶石的过饱和状态由初级生产者分解,并伴生有机质降解,在微环境中触发 | 开阔海洋及浅海陆棚带受光照、温度和营养盐驱动形成生物繁育核心带 | 微晶颗粒,粒径<5 μm,常呈自形微级椭圆体沉淀 |
| 化学 | 海水过饱和条件下Ba2+与SO 自发结合生成微溶硫酸钡沉淀 | 局限海相富Ba2+-SO 水体 | 晶体形态多样,有规则板状、柱状晶体,粒径通常较大 | |
| 成岩型 | I型 | 硫酸盐亏损带富Ba2+流体上涌与海水硫酸盐下渗在硫酸盐—甲烷转换带顶部附近交汇 | 沉积物孔隙水区域 | 晶体较大(>20 μm),扁平板柱状,以铁锰结核、透镜体和纹层等形式分布于沉积物中 |
| II型 | 冷泉区富Ba2+流体与海水SO 垂向交汇低温成矿形成自生硫酸钡 | 冷泉附近,有冷泉流体活动,气液和含甲烷冷溶液 | 晶体较大且不规则,呈脉状、烟囱体、丘状等,如20 m高重晶石丘,重晶石体积占比25%~80% | |
| III型 | 深部富Ba2+热液通过断裂带垂向运移,与海水SO 混合形成硫酸钡 | 洋中脊及弧后盆地等高热液通量区的硫酸盐—硫化物成矿 | 晶体常呈粗大柱状和板状,晶形完整,常伴金属硫化物共生 |
4.1.1 沉积型
4.1.2 成岩型
4.2 非重晶石钡汇
5 生物钡重建古生产力
表4 古生产力重建方法及局限性分析Table 4 Methods and limitations analysis of paleoproductivity reconstruction |
| 重建方法 | 原理 | 局限性 |
|---|---|---|
| 过剩钡(Baexcess)[46,67-70] | 总钡含量减去碎屑铝硅酸盐贡献
式中: 为沉积物中总钡含量; 为沉积物中铝元素含量 | ①生物因素复杂:物种特异性差异,生长阶段及生理状态,死亡后成岩改造; ②环境因素多样:化学条件,环流与水团混合,钡源及迁移复杂; ③分析方法和数据解释不确定:参数关系不明,校准关系适用性受限; ④地质样品干扰剔除不彻底; ⑤假设模型局限性,无法全面适配 |
| 重晶石积累速率 |
式中: (Mass Accumulation Rate)为物质堆积速率; (Dry Bulk Density)为干体积密度,指单位体积干沉积物的质量; 为沉积时间 计算重晶石在沉积物中的积累速率 | |
| Ba/Ti值和Ba/Al值[73] | 假设铝、钛主要源于陆源碎屑且通量稳定,通过计算钡与铝、钛比值,排除陆源碎屑干扰,凸显生物源钡信号 | |
| 综合多元素比值[74] | 分析元素(钡、磷、铝、钛、钙等)比值作为输出生产代理指标 | |
| 钡同位素[10,46,75-76] | 过剩钡和沉积物δ138/134Ba正相关 |
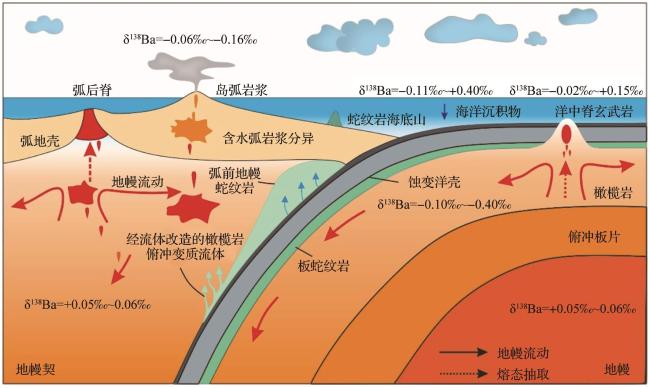
6 非生物钡同位素分馏机制
表5 微观分馏机制汇总Table 5 Summary of microscopic fractionation mechanisms |
| 体系 | 模型及主导因素 | 影响因素 | 数据支撑 | |||
|---|---|---|---|---|---|---|
| 平衡 | 动力学 | 平衡 | 动力学 | 平衡 | 动力学 | |
| 矿物—矿物[77,79] | 质量因素 | 扩散速率、沉淀溶解速率和离子交换速率 | 晶体结构、健长和替代离子 | 温度和浓度 | 重晶石矿物间103lnβ≈0.063[79] | 矿物间扩散β为0.010~0.011[77] |
| 重晶石—流体[68,77-78,80-82] | Ba2+与SO 形成特定配位键,吸附 | 离子交换 | 配位、健长和结构 | 固液比 | 沉淀α precip=0.99968±0.00002,溶解α diss=0.99985±0.00006[77] | 重晶石扩散实验钡β因子在0.010~0.011[77] |
| 毒重石—流体[19,77-78,80] | 溶液Ba2+结构 | 溶液酸碱度和离子种类 | 晶体结构、配位和健长 | 固液比 | 300 K时△138/134Baminerals-fluid=0.094‰[19] | 沉淀过程(α)=0.99993±0.00004[78] |
| 岩浆—热液[83] | 水化作用、Ba2+水合数和水化壳结构 | 结晶速率 | 钡浓度、Al-Si无序,钡浓度 | 矿物结晶和热液流体 | 103lnβ累计平均值=0.0798±0.005‰[83] | — |
| 地幔熔体—流体[19,84-86] | 力常数和健长 | 地幔部分熔融及熔体—地幔流体作用 | 温度、流体盐度和铝指数 | — | 103lnα 流体-熔体落在-0.62‰~-0.14‰内[84] | — |
|





 甘公网安备62010202000687
甘公网安备62010202000687