

Oxidizing Materials Involving in Thermochemical Oxidation of Hydrocarbons in the Lower Triassic Baikouquan Formation, Junggar Basin
Received date: 2020-10-16
Revised date: 2021-03-29
Online published: 2021-11-19
Supported by
the National Natural Science Foundation of China Youth Project "Differences and mechanism of thermochemical oxidation of hydrocarbons in the Lower Triassic clastic reservoir of the Mahu Sag, Junggar Basin"(41902137);The Hunan Natural Science Foundation Youth Project "Reaction sequence and mechanism of thermochemical oxidation of hydrocarbons in deep buried clastic reservoir"(2020JJ5703)
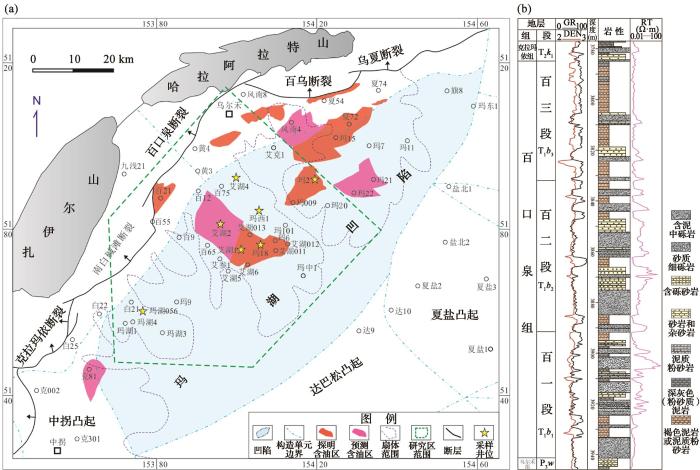
Mechanism of Thermochemical Oxidation of Hydrocarbons (TOH) in the clastic strata of sedimentary basins is unclear.In particular, it is urgently needed to clarify the oxidizing substances involved in the reaction and their state of occurrence before and after the reaction. A related study was carried out in the Lower Triassic Baikouquan (T1b) Formation in Junggar Basin, where the TOH reaction has been confirmed in recent years. Based on detailed core logging, systematic petrological and mineral identification, major elements ofbulk rock and in situ area, and chemical valence analysis of iron ions were carried out. The results show that the cores of this formation show obviously irregular distribution of brown and gray-grayish green layers, and the color changesare not limited in lithologic interface and sedimentary structure. Gray-grayish green layers are most likely the products of bleaching of reducible oil-gas bearing fluid during burial diagenesis. Mineral changes indicate that the color transformation from brown to gray-grayish green is the result of the consumption of oxidizing hematite and the generation of chlorite in the rock. Hematite contains a large amount of Fe3+ and a small amount of Mn3+/4+ ions replacing Fe3+ by isomorphism.In the T1b formation both Fe3+ and Mn3+/4+ in hematite provide effective electron acceptor for the TOH reaction. Mn3+/4+ is preferentially involved in reactions, however,the high content of Fe3+ was the main electron acceptor, followed by low content of Mn3+/4+. The content difference of Fe3+ and Mn3+/4+ causes the fact that the reduction product of authigenic chlorite is rich in Fe, whereas Mn is mainly enriched in low content authigenic calcite cements with extremely negative δ13C values. Abundant hematite participated in the reaction, indicating that the TOH reaction may occur generally in the deep layers of continental petroliferous basins. This reaction will causealterations in mineral composition, remoldingreservoir rocks, and meanwhile consuming large amounts of hydrocarbons in the crust. It is necessary to carry out further studies on this process, particularly in sedimentary basins and subduction zone.
Xun KANG , Ruipu HU , Wenxuan HU , Jingqiang TAN . Oxidizing Materials Involving in Thermochemical Oxidation of Hydrocarbons in the Lower Triassic Baikouquan Formation, Junggar Basin[J]. Advances in Earth Science, 2021 , 36(10) : 1004 -1014 . DOI: 10.11867/j.issn.1001-8166.2021.019
| 1 | KROUSE H R, VIAU C A, ELIUK L S, et al. Chemical and isotopic evidence of thermochemical sulphate reduction by light hydrocarbon gases in deep carbonate reservoirs[J]. Nature, 1988, 333(6 172): 415. |
| 2 | SURDAM R C, JIAO ZHANSHI, MACGOWAN D B. Redox reactions involving hydrocarbons and mineral oxidants: a mechanism for significant porosity enhancement in sandstones[J]. AAPG Bulletin, 1993, 77(9): 1 509-1 518. |
| 3 | WORDEN R H, SMALLEY P C. H2S-producing reactions in deep carbonate gas reservoirs: Khuff Formation, Abu Dhabi[J]. Chemical Geology, 1996, 133(1/4): 157-171. |
| 4 | SEEWALD J S. Organic-inorganic interactions in petroleum-producing sedimentary basins[J]. Nature, 2003, 426(6 964): 327. |
| 5 | GALIMOV E M. Isotope organic geochemistry[J]. Organic Geochemistry, 2006, 37(10): 1 200-1 262. |
| 6 | PAN Changchun, YU Linping, LIU Jinzhong, et al. Chemical and carbon isotopic fractionations of gaseous hydrocarbons during abiogenic oxidation[J]. Earth and Planetary Science Letters, 2006, 246(1/2): 70-89. |
| 7 | HU Wenxuan, KANG Xun, CAO Jian, et al. Thermochemical oxidation of methane induced by high-valence metal oxides in a sedimentary basin[J]. Nature Communications, 2018, 9(5 131): 3-8. |
| 8 | KIYOSU Y, IMAIZUMI S. Carbon and hydrogen isotope fractionation during oxidation of methane by metal oxides at temperatures from 400 to 530 ℃[J]. Chemical Geology, 1996, 133(1/4): 279-287. |
| 9 | STOBBE E R, DE BOER B A, GEUS J W. The reduction and oxidation behaviour of manganese oxides[J]. Catalysis Today, 1999, 47(1/4): 161-167. |
| 10 | SEEWALD J S. Aqueous geochemistry of low molecular weight hydrocarbons at elevated temperatures and pressures: constraints from mineral buffered laboratory experiments[J]. Geochimica et Cosmochimica Acta, 2001, 65(10): 1 641-1 664. |
| 11 | TANG Yong, XU Yang, LI Yazhe, et al. Sedimentation model and exploration significance of large-scaled shallow retrogradation fan delta in Mahu Sag[J]. Xinjiang Petroleum Geology, 2018, 39(1): 16-21. |
| 11 | 唐勇, 徐洋, 李亚哲,等. 玛湖凹陷大型浅水退覆式扇三角洲沉积模式及勘探意义[J]. 新疆石油地质, 2018, 39(1): 16-21. |
| 12 | KANG Xun, HU Wenxuan, CAO Jian, et al. Relationship between hydrocarbon bearing fluid and the differential corrosion of potash feldspar and albite: a case of Baikouquan Formation in Aihu oilfield, Junggar Basin[J]. Acta Petrolei Sinica, 2016, 37(11): 1 383. |
| 12 | 康逊, 胡文瑄, 曹剑, 等. 钾长石和钠长石差异溶蚀与含烃类流体的关系——以准噶尔盆地艾湖油田百口泉组为例[J]. 石油学报, 2016, 37(11): 1 383. |
| 13 | TANG Yong, XU Yang, QU Jianhua, et al. Fan-delta group characteristics and its distribution of the Triassic Baikouquan reservoirs in Mahu Sag of Junggar Basin[J]. Xinjiang Petroleum Geology, 2014, 35(6): 629-633. |
| 13 | 唐勇, 徐洋, 瞿建华,等. 玛湖凹陷百口泉组扇三角洲群特征及分布[J]. 新疆石油地质, 2014, 35(6): 629-633. |
| 14 | CARROLL A R, GRAHAM S A, HENDRIX M S, et al. Late Paleozoic tectonic amalgamation of northwestern China: sedimentary record of the northern Tarim, northwestern Turpan, and southern Junggar basins[J]. GSA Bulletin, 1995, 107(5): 571-594. |
| 15 | CAI Zhongxian, CHEN Fajing, JIA Zhenyuan. Types and tectonic evolut ion of Junger Basin[J]. Earth Science Frontiers, 2000, 7(4): 431-440. |
| 15 | 蔡忠贤, 陈发景, 贾振远. 准噶尔盆地的类型和构造演化[J]. 地学前沿, 2000, 7(4): 431-440. |
| 16 | TANG Yong, GUO Wenjian, WANG Xiatian, et al. A new breakthrough in exploration of large conglomerate oil province in Mahu Sag and its implications[J]. Xinjiang Petroleum Geology, 2019, 40(2): 127-135. |
| 16 | 唐勇, 郭文建, 王霞田,等. 玛湖凹陷砾岩大油区勘探新突破及启示[J]. 新疆石油地质, 2019, 40(2): 127-135. |
| 17 | JIA Haibo, JI Hancheng, LI Xinwei, et al. A retreating fan-delta system in the Northwestern Junggar Basin, northwestern China—characteristics, evolution and controlling factors[J]. Journal of Asian Earth Sciences, 2016, 123: 162-177. |
| 18 | HAYNES W M. CRC handbook of chemistry and physics[M]. Florida: CRC Press, 2014: 12 100-12 236. |
| 19 | ALVAREZ M, RUEDA E H, SILEO E E. Simultaneous incorporation of Mn and Al in the goethite structure[J]. Geochimica et Cosmochimica Acta, 2007, 71(4): 1 009-1 020. |
| 20 | LIU Huan, LU Xiancai, LI Juan, et al. Geochemical fates and unusual distribution of arsenic in natural ferromanganese duricrust[J]. Applied Geochemistry, 2017, 76: 74-87. |
| 21 | ARTAMONOVA I V, GORICHEV I G, GODUNOV E B. Kinetics of manganese oxides dissolution in sulphuric acid solutions containing oxalic acid[J]. Engineering, 2013, 5(9): 714. |
| 22 | WALANDA D K, LAWRANCE G A, DONNE S W. Hydrothermal MnO2: synthesis, structure, morphology and discharge performance[J]. Journal of Power Sources, 2005, 139(1/2): 325-341. |
| 23 | HEIN J R, KOSKI R A. Bacterially mediated diagenetic origin for chert-hosted manganese deposits in the Franciscan Complex, California Coast Ranges[J]. Geology, 1987, 15(8): 722-726. |
| 24 | BEAL E J, HOUSE C H, ORPHAN V J. Manganese-and iron-dependent marine methane oxidation[J]. Science, 2009, 325(5 937): 184-187. |
| 25 | SIVAN O, ANTLER G, TURCHYN A V, et al. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps[J]. Proceedings of the National Academy of Sciences, 2014, 111(40): E4139-E4147. |
| 26 | CHEN Qilin, HUANG Chenggang. Research progress of modification of reservoirs by dissolution in sedimentary rock[J]. Advances in Earth Science, 2018, 33(11): 1 112-1 129. |
| 26 | 陈启林, 黄成刚. 沉积岩中溶蚀作用对储集层的改造研究进展[J]. 地球科学进展, 2018, 33(11): 1 112-1 129. |
| 27 | DU Jiangmin, LONG Pengyu, YANG Peng, et al. Characteristics of carbonate reservoir and its forming conditions in continental lake basin of China[J]. Advances in Earth Science, 2020, 35(1): 52-69. |
| 27 | 杜江民, 龙鹏宇, 杨鹏, 等. 中国陆相湖盆碳酸盐岩储集层特征及其成藏条件[J]. 地球科学进展, 2020, 35(1): 52-69. |
/
| 〈 |
|
〉 |