Utilization and exploitation of lithium resources in salt lakes and some suggestions concerning development of Li industries in China
1
20146
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
盐湖锂资源开发利用及对中国锂产业发展的建议
1
2014
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Global lithium resources: Relative importance of pegmatite, brine and other deposits
3
2012
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... [2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... [2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Evaporites through time: Tectonic, climatic and eustatic controls in marine and nonmarine deposits
1
2010
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Geological features and metallogenic model of K- and Li-rich brine ore field in the Jiangling Depression
2
2018
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
江陵凹陷富钾锂卤水矿田地质特征及成藏模式研究
2
2018
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
The metallogenetic regularity of lithium deposit in China
1
2014
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
中国锂矿成矿规律概要
1
2014
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Analysis of the global lithium resource distribution and potential
1
2015
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
全球锂资源分布与潜力分析
1
2015
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
A tentative discussion on regional metallogenic background and mineralization mechanism of subterranean brines rich in potassium and lithium in South Chian Block
1
2016
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
华南陆块液体钾锂资源的区域成矿背景与成矿作用初探
1
2016
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
The main types, distribution features and present situation of exploration and development for domestic and foreign lithium mine
1
2017
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
国内外锂矿主要类型、分布特点及勘查开发现状
1
2017
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Exploitation of lithium in salt lakes and environment
1
2000
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
青海盐湖锂盐开发与环境
1
2000
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Saline lake lithium resources of China and its exploitation
1
2003
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
中国盐湖锂资源及其开发进程
1
2003
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Exploitation actuality of saline lake lithium resources in Tibet
1
2004
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
西藏地区盐湖锂资源的开发现状
1
2004
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Status of development of potassium resource in Cha-er-han salt lake in Qinghai
1
2009
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
青海察尔汗盐湖资源开发现状
1
2009
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Lithium resources industrialization of salt lakes in China: A case study of the Xitaijinaier salk lake and the Zhabuye salt lake
1
2010
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
中国盐湖锂资源的产业化现状——以西台吉乃尔盐湖和扎布耶盐湖为例
1
2010
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
S. Geological Survey Open-File Report
1
2013
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
A review of Australian salt lakes and associated mineral systems
1
2016
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Development and utilization of potash resources of saline lakes in Qinghai Province
1
2017
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
青海盐湖钾盐资源开发利用及产业发展
1
2017
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
Lithium and strontium isotopic systematics in playas in Nevada, USA: Constraints on the origin of lithium
2
2013
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
The role of climate in the accumulation of lithium-rich brine in the Central Andes
5
2013
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Stable isotopes (Li, O, H) combined with brine chemistry: Powerful tracers for Li origins in Salar deposits from the Puna region, Agentina
2
2015
... 锂(Li)元素位于元素周期表第Ⅰ主族,是最轻的金属元素,具有高热容量、高电离能,且在碱金属元素中,其具有高熔点和沸点.Li元素被广泛应用于陶瓷、电子与电气、冶金、医疗和光学等行业.近20年来,Li成为一种战略性新兴矿产资源,是制造新能源汽车电池的重要原料[1].早期,Li资源主要来自岩浆成因的伟晶岩矿床,少量来自蒸发岩[2].目前,卤水型锂矿已成为Li资源的主要来源[2,3,4,5,6,7,8].卤水型锂矿可进一步分为盐湖卤水型和深层地下卤水型锂矿,世界上主要的卤水型锂矿包括美国的西尔斯湖、索尔顿海湖盐湖卤水和克莱顿峡谷地下卤水、玻利维亚的乌尤尼盐湖、智利的阿塔卡玛盐湖、阿根廷的翁布雷穆埃尔托盐湖、澳大利亚盐湖以及中国西藏的扎布耶和当雄错盐湖、青海的察尔汗和台吉乃尔盐湖[2,9,10,11,12,13,14,15,16].基于南美和北美西部高原地区地下富Li卤水Li同位素研究[17,18,19],表明地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium isotope geochemistry
1
2017
... Li有2个稳定同位素,分别为7Li(92.4%)和6Li(7.6%),它们之间的相对质量差高达约17%.由于7Li和6Li扩散的差异性,不同环境条件下,Li同位素值呈现不同的变化范围.在低温条件下,Li同位素值组成变化跨度可超过50‰;高温条件下,Li同位素值的组成变化也是非常大的[20].过去Li同位素值的表示方法用δ6Li表示,目前国际上已使用δ7Li来表示: ...
A secondary isotopic standard for 6Li/7Li determinations
1
1973
... 国际上一直使用L-SVEC(碳酸锂)作为Li同位素标准参考物质[21],但这种标样不再生产,因此,IRMM-016(碳酸锂)作为一个理想的标准物质替代标样L-SVEC[22]. ...
Calibrated measurements of the isotopic composition and atomic weight of the natural Li isotopic reference material IRMM-016
1
1997
... 国际上一直使用L-SVEC(碳酸锂)作为Li同位素标准参考物质[21],但这种标样不再生产,因此,IRMM-016(碳酸锂)作为一个理想的标准物质替代标样L-SVEC[22]. ...
Variation of lithium isotope composition in the marine environment: A preliminary report
3
1988
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium isotopic composition of submarine basalts: Implications for the lithium cycle in the oceans
3
1992
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... [24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Across-arc variation of Li isotopes in lavas and implications for crust/mantle recycling at subduction zones
2
1998
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... [25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotope evidence for light element decoupling in the Panama subarc mantle
1
2000
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotopic composition of Central American volcanic arc lavas: Implications for modification of subarc mantle by slab-derived fluids: Correction
2
2002
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 由于地下水样品中其他元素(如Na、Mg、Ca等)含量是Li元素含量的数百甚至数千倍,所以Li元素的分离与纯化是一项极具挑战性的工作.目前,比较成熟的方法是阳离子交换色谱法,利用Li特效树脂(AG50W-X8或AG50W-X12)分离洗脱.常用的淋洗液有HCl、HNO3、HCl+乙醇以及HNO3+甲醇,在Li被洗出的过程中,会发生Li同位素的分馏[77],需保证百分之百的Li回收率.并且,不同的样品在Li的分离纯化过程中其析出峰值位置是不同的[27,39],因此不同的样品需要对离子交换柱进行矫正.相对于主量元素K、Ca和Mg来说,由于Na与Li具有相似的化学性质,它们之间的分离是最困难的,如果分离不当就会出现Na的大量拖尾,导致分离纯化的失败. ...
Boron and lithium isotopic variations in a hot subduction zone—The southern Washington Cascades
1
2004
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium nucleosynthesis in the Sun inferred from the solar-wind 7Li/6Li ratio
1
1999
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
An ultra high-resolution survey of the interstellar 7Li/6Li isotope ratio in the solar neighborhood
1
2003
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Measurements of the isotopic ratio 6Li/7Li in stars with planets
1
2009
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
The lithium isotopic ratio in very metal-poor stars
1
2013
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotopic compositioin of the dissolved and suspended loads of the Yangtze River, China
2
2008
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
长江流域河水和悬浮物的锂同位素地球化学研究
2
2008
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Behavior of lithium isotopes in the Changjiang River system: Sources effects and response to weathering and eosion
1
2015
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes
1
2015
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotope behavior during weathering in the Ganges Alluvial Plain
1
2017
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotope study of hot springs and metabasalts from Mid-Ocean Ridge Hydrothermal Systems
3
1993
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
Lithium isotopes in island arc geothermal systems: Guadeloupe, Martinique (French West Indies) and experimental approach
10
2010
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... ];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 数据来源:海底热液来自参考文献[38,106,107];大陆地热水来自参考文献[18,38,100~103,105] ...
... 近年来,随着Li同位素地球化学在地热系统中的应用研究,发现储层温度控制着地热水的Li同位素组成,即它们之间存在较好的相关性[38,44,101].对法国中央高原地区地热储库的Li-B-Sr多同位素体系研究[101]发现,相对B和Sr同位素变化范围,Li同位素值显示了更窄的波动,并且很少依赖于储层岩性特征.进一步利用地热储层温度估算公式计算出的温度与地热流体的δ7Li值投图,建立了储层温度和δ7Li值之间的相关性方程,即δ7Li=(-0.043±0.003)T+(11.9±0.5).这些结果表明地热水的Li同位素组成可以作为地质温度计.Millot等[38]完成了海水与玄武岩在25~250 ℃范围的水岩反应实验,建立了Li同位素分馏值与温度之间的经验关系式,即Δsolution-solid=7 847/T-8.093.并用这个经验关系式计算了马提尼克岛和瓜德罗普岛火山岛弧区域地热体系的储层温度,这些温度结果与地热水溶质温度估算公式计算的一致,表明了Li同位素体系是一个用于地热储层温度计算的有利工具.Millot等[44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... [38]完成了海水与玄武岩在25~250 ℃范围的水岩反应实验,建立了Li同位素分馏值与温度之间的经验关系式,即Δsolution-solid=7 847/T-8.093.并用这个经验关系式计算了马提尼克岛和瓜德罗普岛火山岛弧区域地热体系的储层温度,这些温度结果与地热水溶质温度估算公式计算的一致,表明了Li同位素体系是一个用于地热储层温度计算的有利工具.Millot等[44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
Lithium isotopic composition of Central American Volcanic Arc lavas: Implications for modification of subare mantle by slab-derived fluids
2
1999
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 由于地下水样品中其他元素(如Na、Mg、Ca等)含量是Li元素含量的数百甚至数千倍,所以Li元素的分离与纯化是一项极具挑战性的工作.目前,比较成熟的方法是阳离子交换色谱法,利用Li特效树脂(AG50W-X8或AG50W-X12)分离洗脱.常用的淋洗液有HCl、HNO3、HCl+乙醇以及HNO3+甲醇,在Li被洗出的过程中,会发生Li同位素的分馏[77],需保证百分之百的Li回收率.并且,不同的样品在Li的分离纯化过程中其析出峰值位置是不同的[27,39],因此不同的样品需要对离子交换柱进行矫正.相对于主量元素K、Ca和Mg来说,由于Na与Li具有相似的化学性质,它们之间的分离是最困难的,如果分离不当就会出现Na的大量拖尾,导致分离纯化的失败. ...
Lithium isotopic systematics of peridotite xenoliths from Hannuoba, North China Craton: Implications for melt-rock interaction in the considerably thinned lithospheric mantle
1
2007
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
The origin and evolution of Canadian Shield brines: Evaporation or freezing of seawater?New lithium isotope and geochemical evidence from the Slave craton
2
1999
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium isotope geochemistry and origin of Canadian shield brines
1
2003
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Multi-isotopic (Li, B, Sr, Nd) approach for geothermal reservoir characterization in the Limagne Basin (Massif Central, France)
9
2007
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... ,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... [43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... [43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Chemical, multi-isotopic (Li-B-Sr-U-H-O) and thermal characterization of Triassic formation waters from the Paris Basin
14
2011
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... 近年来,随着Li同位素地球化学在地热系统中的应用研究,发现储层温度控制着地热水的Li同位素组成,即它们之间存在较好的相关性[38,44,101].对法国中央高原地区地热储库的Li-B-Sr多同位素体系研究[101]发现,相对B和Sr同位素变化范围,Li同位素值显示了更窄的波动,并且很少依赖于储层岩性特征.进一步利用地热储层温度估算公式计算出的温度与地热流体的δ7Li值投图,建立了储层温度和δ7Li值之间的相关性方程,即δ7Li=(-0.043±0.003)T+(11.9±0.5).这些结果表明地热水的Li同位素组成可以作为地质温度计.Millot等[38]完成了海水与玄武岩在25~250 ℃范围的水岩反应实验,建立了Li同位素分馏值与温度之间的经验关系式,即Δsolution-solid=7 847/T-8.093.并用这个经验关系式计算了马提尼克岛和瓜德罗普岛火山岛弧区域地热体系的储层温度,这些温度结果与地热水溶质温度估算公式计算的一致,表明了Li同位素体系是一个用于地热储层温度计算的有利工具.Millot等[44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... [44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... [44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... [44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... [44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... ,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... [44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Chemical weathering processes in the Great Artesian Basin: Evidence from lithium and silicon isotopes
1
2014
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Origins of lithium in submarine mud volcano fluid in the Nankai accretionary wedge
2
2015
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 地热水的运移需要一定动能,而地下热能的变化可以驱动地热流体的流动与运移,特别是火山区以及汇聚大陆边缘地热水的运移更加频繁.与传统同位素相比,Li同位素可以作为有效的工具预测深部地热流体流动与地震活动的关系.Nishio等[105]调查了日本中部火山地区地热水的Li同位素,发现来自地震震中的地热水样品以低的δ7Li值(-5.17‰~1.55‰)为特征,显示了下地壳深部流体的Li同位素组成性质,表明了Li来自下地壳,而其他地震不活跃地区的地热水样品呈现了更高的δ7Li值,显示了地热水与上地壳岩石水岩反应所呈现的Li同位素组成,代表了上地壳Li的信息.因此,这些以低δ7Li值为特征的地热流体来自下地壳深部地震的活动.当然,地热水低的δ7Li值也可以解释为一定温度下流体与岩石间Li同位素扩散交换的结果[102,103,120].然而,Nishio等[46]利用日本Nankai增生楔泥火山中流体的Li同位素特征成功证明了深部流体流动和地震活动间的联系. ...
Lithium isotopes as tracers of groundwater circulation in a peat land
1
2010
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Lithium isotope systematics in a forested granitic catchment (Strengbach, Vosges Mountains, France)
1
2010
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Processes controlling the stable isotope compositions of Li, B, Mg and Ca in plants, soils and waters: A review
1
2012
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Classification of Geothermal Field and Geothermal Geology Characteristics in Nanyang Basin
1
2011
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
南阳盆地地热田类型划分及地热地质特征
1
2011
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Study on Method and Application of Economic Evaluation of Geothermal Resources
1
2013
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
地热资源经济评价方法与应用研究
1
2013
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
A tentative discussion on an evaluation system of geothermal unit ranking and classification in China
1
2017
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
试论中国地热单元分级分类评价体系
1
2017
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
Hydrogeochemistry of high-temperature geothermal systems in China: A review
2
2012
... Li同位素的分馏不仅发生在地表或近地表[23],而且在海水与玄武岩之间[24],甚至在更深的俯冲带均可发生[25,26,27,28].随着分析测试技术的快速发展,Li同位素地球化学在宇宙行星[29,30,31,32]、大陆风化[33,34,35,36]、洋壳蚀变与海底热液[24,37,38]、板块俯冲与壳幔演化[25,39,40]、流体的来源与演化[41,42,43,44,45,46]以及生物与人类行为[47,48,49]等领域取得了显著成果.地热是一种洁净且可再生的天然资源,在世界上已被广泛地用于发电、取暖和温室养殖等方面[50].按地质构造成因特征,可分为沉积盆地型和隆起山地型地热资源[51].地热体系是相对独立的热能生成、运移、聚集和保持的自然系统,是一个相对独立的单元,是地热形成机理研究的重点[52].水化学和传统稳定同位素已在地热水形成机理研究中广泛应用[53],相对而言,在中国非传统Li同位素在地热水成因研究中较为薄弱.此外,大陆地热体系由于岩石的复杂性而较少受到关注,且大陆地热活动中的Li同位素行为尚未得到较好的认识.为更好地呈现国际上对大陆地热体系Li同位素地球化学研究成果,本文归纳和总结了大陆地热体系中Li同位素组成及其应用前景,并对该研究领域的未来提出展望和建议,以期为未来大陆地热体系的研究以及资源的开发、利用提供科学的参考. ...
... 不同的地质构造背景可形成不同类型的地热系统,首先,各类型地热体系Li同位素组成背景及其时空分布特征是怎样的尚不清楚,其次,这些体系内地热流体循环运移过程中Li同位素行为也是不清楚的,制约着Li同位素在大陆地热体系中的应用研究.大陆地热储库岩石主要为碎屑岩、碳酸盐岩和火成岩等[53,119,131],由于储库岩石的复杂性,其主要问题是地热流体与岩石间水岩反应过程中主要矿物相溶解和次生矿物形成机制是不清楚的,且该过程中Li同位素分馏机制还无法得到清楚的解释.这就需要我们注重不同温度下的水岩反应实验研究,定量化不同温度下次生矿物形成或Li被吸附于矿物表面所导致的Li同位素分馏机制.因此,将来在该方面的工作研究对地热体系中Li 的成因与演化的解释具有重要的意义.同时,我们也应该关注储层岩石的矿物学研究,地球化学与矿物学的双重验证才能更好地揭示地质作用过程. ...
The thermodynamic properties of isotopic substances
1
1947
... 平衡热力学和相关实验研究表明Li同位素分馏有温度依赖性.随着温度的降低,两相间的分馏系数会渐渐发生变化[54].这种性质被解释为键能控制的7Li和6Li在两相间的配分,而配位数的高低决定了键能的高低,配位数越低,键能越高,7Li优先进入高键能的配位点[55].具体可分为高温和低温条件下的同位素分馏机制. ...
Applying stable isotope fractionation theory to new systems
1
2004
... 平衡热力学和相关实验研究表明Li同位素分馏有温度依赖性.随着温度的降低,两相间的分馏系数会渐渐发生变化[54].这种性质被解释为键能控制的7Li和6Li在两相间的配分,而配位数的高低决定了键能的高低,配位数越低,键能越高,7Li优先进入高键能的配位点[55].具体可分为高温和低温条件下的同位素分馏机制. ...
Temperature-dependent isotopic fractionation of lithium between clinopyroxene and high-pressure hydrous fluids
1
2006
... Wunder等[56]分析了在2.0 GPa压力条件下,500~900 ℃温度变化范围内,锂辉石与碱性、酸性流体间的Li同位素分馏过程.实验表明,7Li优先进入流体相,且在一定压力条件下,随着温度升高,辉石与流体间的分馏系数渐渐变小.Wunder等[57]调查了蛇纹石和流体间的同位素分馏(200~500 ℃),研究发现,含Li的蛇纹石(叶蛇纹石和利蛇纹石)与流体间的Li同位素分馏显示了与锂云母(300~500 ℃)[58]相似的分馏机制,进一步证实了7Li优先进入流体相,相对于固相,流体呈现了高的Li同位素值.然而,在十字石、纤维蛇纹石与流体间的同位素分馏实验中[57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
The effect of chrysotile nanotubes on the serpentine-fluid Li-isotopic fractionation
2
2010
... Wunder等[56]分析了在2.0 GPa压力条件下,500~900 ℃温度变化范围内,锂辉石与碱性、酸性流体间的Li同位素分馏过程.实验表明,7Li优先进入流体相,且在一定压力条件下,随着温度升高,辉石与流体间的分馏系数渐渐变小.Wunder等[57]调查了蛇纹石和流体间的同位素分馏(200~500 ℃),研究发现,含Li的蛇纹石(叶蛇纹石和利蛇纹石)与流体间的Li同位素分馏显示了与锂云母(300~500 ℃)[58]相似的分馏机制,进一步证实了7Li优先进入流体相,相对于固相,流体呈现了高的Li同位素值.然而,在十字石、纤维蛇纹石与流体间的同位素分馏实验中[57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
... [57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
Lithium isotope fractionation between Li-bearing staurolite, Li-mica and aqueous fluids: An experimental study
2
2007
... Wunder等[56]分析了在2.0 GPa压力条件下,500~900 ℃温度变化范围内,锂辉石与碱性、酸性流体间的Li同位素分馏过程.实验表明,7Li优先进入流体相,且在一定压力条件下,随着温度升高,辉石与流体间的分馏系数渐渐变小.Wunder等[57]调查了蛇纹石和流体间的同位素分馏(200~500 ℃),研究发现,含Li的蛇纹石(叶蛇纹石和利蛇纹石)与流体间的Li同位素分馏显示了与锂云母(300~500 ℃)[58]相似的分馏机制,进一步证实了7Li优先进入流体相,相对于固相,流体呈现了高的Li同位素值.然而,在十字石、纤维蛇纹石与流体间的同位素分馏实验中[57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
... ,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
Lithium speciation in aqueous fluids at high P and T studied by ab initio molecular dynamics and consequences for Li-isotope fractionation between minerals and fluids
1
2009
... Wunder等[56]分析了在2.0 GPa压力条件下,500~900 ℃温度变化范围内,锂辉石与碱性、酸性流体间的Li同位素分馏过程.实验表明,7Li优先进入流体相,且在一定压力条件下,随着温度升高,辉石与流体间的分馏系数渐渐变小.Wunder等[57]调查了蛇纹石和流体间的同位素分馏(200~500 ℃),研究发现,含Li的蛇纹石(叶蛇纹石和利蛇纹石)与流体间的Li同位素分馏显示了与锂云母(300~500 ℃)[58]相似的分馏机制,进一步证实了7Li优先进入流体相,相对于固相,流体呈现了高的Li同位素值.然而,在十字石、纤维蛇纹石与流体间的同位素分馏实验中[57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
Li-isotope fractionation between silicates and fluids: Pressure dependence and influence of the bonding environment
2
2011
... Wunder等[56]分析了在2.0 GPa压力条件下,500~900 ℃温度变化范围内,锂辉石与碱性、酸性流体间的Li同位素分馏过程.实验表明,7Li优先进入流体相,且在一定压力条件下,随着温度升高,辉石与流体间的分馏系数渐渐变小.Wunder等[57]调查了蛇纹石和流体间的同位素分馏(200~500 ℃),研究发现,含Li的蛇纹石(叶蛇纹石和利蛇纹石)与流体间的Li同位素分馏显示了与锂云母(300~500 ℃)[58]相似的分馏机制,进一步证实了7Li优先进入流体相,相对于固相,流体呈现了高的Li同位素值.然而,在十字石、纤维蛇纹石与流体间的同位素分馏实验中[57,58,59],发现截然相反的Li同位素分馏,即矿物有高于流体的δ7Li值.这种同位素分馏行为被解释为不同矿物中Li的配位数不同所造成的,Li在十字石和纤维蛇纹石中的配位数小于流体的,键能高,而7Li优先进入高键能的低配位点.同时,Wunder等[60]发现尽管Li在锂辉石和锂云母中均为六面体配位,但它们与流体间显示了不同的同位素分馏行为.显然,这不能用配位数差异来解释,而是由于键价的不同而产生的.在相同配位数的条件下,平均Li-O键较短的矿物具有较大的Li总键价,而7Li优先进入高Li总键价的矿物中.因此,由于锂云母总键价高于锂辉石,所以锂云母与流体间的同位素分馏小于锂辉石与流体间的同位素分馏. ...
... 除温度外,压力对矿物与流体间的Li同位素分馏的影响也是非常重要的.Wunder等[60]测量了在500~625 ℃温度范围与1、4和8 GPa压力条件下锂辉石与流体间的分馏值(Δ7Lisolid-fluid).结果表明,在1和4 GPa压力条件下,Δ7Lisolid-fluid值约为-3.5‰,压力并未对其分馏产生影响,而在8 GPa压力条件下,Δ7Lisolid-fluid值约为-1.9‰. ...
Lithium isotope geochemistry of sediments and hydrothermal fluids of the Guaymas Basin, Gulf of California
2
1994
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
Lithium isotope geochemistry of pore waters from Ocean Drilling Program Sites 918 and 919, Irminger Basin
1
1988
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Lithium-isotope fractionation during continental weathering processes
3
2003
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... [63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina
1
2004
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Quantifying Li isotope fractionation during smectite formation and implications for the Li cycle
2
2008
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... [65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Lithium isotope fractionation during uptake by gibbsite
2
2015
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
... [66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Lithium and boron isotopes in illite-smectite: The importance of crystal size
1
2005
... 前人对低温条件下的Li同位分馏机制进行了大量研究,其分馏机制主要归因于次生矿物形成过程中的吸附或并入作用等[24,37,61,62,63,64,65,66].三水铝矿、蒙脱石和氢氧化铁胶体与流体间因吸附作用而发生的Li同位素分馏被实验所证实[63].研究发现,在22 ℃温度下,三水铝矿的吸附作用可使流体的δ7Li值高出初始流体8‰~12‰,分馏系数α三水铝矿—流体为0.986.其次,氢氧化铁胶体吸附作用产生了一定的同位素分馏,而蒙脱石吸附作用几乎不发生同位素分馏.Wimpenny等[66]认为三水铝矿与流体间的Li同位素分馏可以分为2个阶段:首先是三水铝矿结构的扩张造成Li扩散进入层间和充填八面体空位,导致流体与三水铝矿的同位素分馏值可达16‰(α三水铝矿—流体约为0.984);其次,当八面体空位被充填完毕后,流体与三水铝矿间的同位素分馏微弱.在低温条件下(25~90 ℃),蒙脱石的分馏系数变化很小,从90 ℃时的0.990降低到25 ℃的0.983[65].这种现象被解释为低温条件下黏土矿物贫穷结晶的结果,因为这些贫穷结晶的黏土晶体有大比例的共棱八面体,会造成不同程度的Li同位素分馏.同时,在成岩作用阶段,蒙脱石向伊利石转化时也会发生Li同位素分馏.在蒙脱石向伊利石转化的实验中,新生成的不同粒级的黏土矿物有不同的Li同位素组成,且粗粒级(>2 μm)的黏土与流体间的Li同位素分馏值可达11‰[67]. ...
Lithium elemental and isotopic disequilibrium in minerals from peridotite xenoliths from far-east Russia: Product of recent melt/fluid-rock reaction
1
2007
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
Comparative diffusion coefficients of major and trace elements in olivine at ~950℃ from a xenocryst included in dioritic magma
1
2010
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
Rates of Li diffusion in garnet: Coupled transport of Li and Y plus REEs
1
2014
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
Lithium isotope fractionation by diffusion in minerals. Part 1: Pyroxenes
1
2014
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
High-temperature lithium isotope fractionation: Insights from lithium isotope diffusion in magmatic systems
1
2007
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
Diffusion of Li in olivine. Part I: Experimental observations and a multi species diffusion model
1
2010
... 动力分馏是指在不完整或单向的过程中(例如蒸发或沿一个化学势梯度扩散)一个同位素被运移的速度高于另一个,由于两个同位素扩散系数的差异从而导致同位素的分馏.在矿物或岩石中因扩散性差异所产生的动力分馏被前人研究所证实[68,69,70].辉石矿物内诱导的扩散实验[71]表明,在某种条件下,Li呈现了典型平滑变化的扩散曲线,归因于Li占据了快速扩散的间隙位置;在其他条件下,Li含量呈现了阶梯状变化的曲线,归因于在更加有限的扩散性下Li发生分馏进入金属活性位.同时,橄榄岩中的Li同位素分馏实验数据[72,73]表明,本质上较慢的Li扩散机制起主导地位,然而在橄榄石中Li的平均扩散速率比Fe和Mg的扩散速率高出一个数量级. ...
Molecular dynamics simulations of kinetic isotope fractionation during the diffusion of ionic species in liquid water
1
2007
... 其次,由于温度梯度所发生的元素再分配也会引起同位素分馏[74,75,76].安山岩活塞式液压缸部分熔融实验[75]表明,在冷端(411 ℃)和热端(952 ℃)之间存在Li同位素分馏,分馏值达18.5‰.同样地,Richter等[76]完成了玄武质熔体的高温高压实验,发现冷端(411 ℃)和热端(952 ℃)之间的Li同位素分馏值约为8‰. ...
Stable isotope fractionation by thermal diffusion through partially molten wet and dry silicate rocks
2
2013
... 其次,由于温度梯度所发生的元素再分配也会引起同位素分馏[74,75,76].安山岩活塞式液压缸部分熔融实验[75]表明,在冷端(411 ℃)和热端(952 ℃)之间存在Li同位素分馏,分馏值达18.5‰.同样地,Richter等[76]完成了玄武质熔体的高温高压实验,发现冷端(411 ℃)和热端(952 ℃)之间的Li同位素分馏值约为8‰. ...
... [75]表明,在冷端(411 ℃)和热端(952 ℃)之间存在Li同位素分馏,分馏值达18.5‰.同样地,Richter等[76]完成了玄武质熔体的高温高压实验,发现冷端(411 ℃)和热端(952 ℃)之间的Li同位素分馏值约为8‰. ...
Isotope fractionation of Li and K in silicate liquids by Soret diffusion
2
2014
... 其次,由于温度梯度所发生的元素再分配也会引起同位素分馏[74,75,76].安山岩活塞式液压缸部分熔融实验[75]表明,在冷端(411 ℃)和热端(952 ℃)之间存在Li同位素分馏,分馏值达18.5‰.同样地,Richter等[76]完成了玄武质熔体的高温高压实验,发现冷端(411 ℃)和热端(952 ℃)之间的Li同位素分馏值约为8‰. ...
... [76]完成了玄武质熔体的高温高压实验,发现冷端(411 ℃)和热端(952 ℃)之间的Li同位素分馏值约为8‰. ...
Precise measurementof Li isotopes in planktonic foraminiferal tests by quadrupole ICPMS
1
2001
... 由于地下水样品中其他元素(如Na、Mg、Ca等)含量是Li元素含量的数百甚至数千倍,所以Li元素的分离与纯化是一项极具挑战性的工作.目前,比较成熟的方法是阳离子交换色谱法,利用Li特效树脂(AG50W-X8或AG50W-X12)分离洗脱.常用的淋洗液有HCl、HNO3、HCl+乙醇以及HNO3+甲醇,在Li被洗出的过程中,会发生Li同位素的分馏[77],需保证百分之百的Li回收率.并且,不同的样品在Li的分离纯化过程中其析出峰值位置是不同的[27,39],因此不同的样品需要对离子交换柱进行矫正.相对于主量元素K、Ca和Mg来说,由于Na与Li具有相似的化学性质,它们之间的分离是最困难的,如果分离不当就会出现Na的大量拖尾,导致分离纯化的失败. ...
Lithium isotope analysis by thermal ionization mass spectrometry of lithium tetraborate
1
1987
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
High-yield lithium separation and the precise isotopic analysis for natural rock and aqueous samples
1
1998
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Accurate and precise determination of Li isotopic compositions by multi-collector sector ICP-MS
2
1999
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Precise, small sample size determinations of lithium isotopic compositions of geological reference materials and modern seawater by MC-ICP-MS
1
2004
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Low-blank separation and isotope ratio measurement of small samples of lithium using multiple-collector ICPMS
1
2004
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Accurate and high-precision measurement of lithium isotopes in two reference materials by MC-ICP-MS
2
2004
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium isotope composition of basalt glass reference material
1
2005
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
MPI-DING reference glasses for in situ microanalysis: New reference values for element concentrations and isotope ratios
1
2006
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Isotopic analysis of lithium by atomic absorption spectrophotometry
1
1971
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Determination of isotopic composition of lithium by neutron activation analysis; comparison with mass spectrometry
1
1977
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Determination of 6Li/7Li isotope ratio using two photon resonance three photon resonance ionization mass spectrometry
1
1998
... 目前,能对Li同位素进行精确测量的主要仪器有四级杆电感耦合等离子质谱(Quadrupole Inductively Coupled Plasma Mass Spectrometry,Q-ICPMS)、扇形磁场电感耦合等离子质谱(Sector Field-Inductively Coupled Plasma Mass Spectrometry,SF-ICPMS)、热电离质谱(Thermal Ionization Mass Spectrometry,TIMS)、多接收电感耦合等离子质谱(Multiple-Collector Inductively Coupled Plasma Mass Spectrometry,MC-ICPMS)和二次离子质谱(Secondary Ion Mass Spectrometry,SIMS).在ICPMS和SIMS多元化的发展之前,TIMS是高精度Li同位素测量的主要选择.由于TIMS测样耗时长且不易监控仪器质量偏差的变化等[78,79],近年来,MC-ICPMS受到越来越多学者的青睐,该方法所需样品量少、测样时间短且能避免测试过程中基质效应等因素产生的干扰[80,81,82,83],已成为高精度Li同位素测量的首选方法.SIMS是对样品进行原位Li同位素分析的方法,不需要对样品进行化学分离纯化,但其分析精度较差,且有许多问题要解决[84,85],例如合适标样的选择等,需进一步发展优化.其次,原子吸收光谱(Atomic Absorption Spectrometry, AAS)[86]、中子活化分析(Neutron Activation Analysis, NAA)[87]和共振电离质谱(Resonance Ionization Mass Spectrometry, RIMS)[88]也可进行Li同位素测试分析,但其应用面较窄. ...
Precise lithium isotopic composition in low concentration natural samples
1
1996
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Precise determination of lithium isotopic composition by thermal ionization mass spectrometry in natural samples such as seawater
1
1998
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium and its isotopes in major world rivers: Implications for weathering and the oceanic budget
3
1998
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
91]
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Multi-isotopic composition (δ7Li-δ11B-δD-δ18O) of rain waters in France: Origin and spatio-temporal characterization
3
2010
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
Lithium isotopic compositions of brine, sediments and source water in Da Qaidam Lake, Qinghai, China
5
1994
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93]
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93]
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93,
96]
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
青海柴达木湖卤水、沉积物和水源中锂同位素组成
5
1994
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93]
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93]
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
93,
96]
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Determination of ultra-trace elements in snow samples by inductively-coupled plasma source sector field mass spectrometry using ultrasonic nebulization
1
2000
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Geographical variations of major and trace elements in East Antarctica
1
1999
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes
3
2019
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... ,
96]
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Lithium brines: A global perspective
5
2016
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... [
97]
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Characteristics and formation of potassium-bearing brine in the deeper strata in depression in Hubei Jiangling Province
1
2011
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
湖北江陵凹陷深层高温富钾卤水特征及其成因探讨
1
2011
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
Characteristics and formation of potash deposits in continental rift basins: A review
1
2013
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
大陆裂谷盆地钾盐矿床特征与成矿作用
1
2013
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
The lithium isotopic composition of waters of the Mono Basin, California
4
2003
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
Multi-isotopic tracing (δ7Li, δ11B, 87Sr/86Sr) and chemical geothermometry: Evidence from hydro-geothermal systems in France
11
2007
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 近年来,随着Li同位素地球化学在地热系统中的应用研究,发现储层温度控制着地热水的Li同位素组成,即它们之间存在较好的相关性[38,44,101].对法国中央高原地区地热储库的Li-B-Sr多同位素体系研究[101]发现,相对B和Sr同位素变化范围,Li同位素值显示了更窄的波动,并且很少依赖于储层岩性特征.进一步利用地热储层温度估算公式计算出的温度与地热流体的δ7Li值投图,建立了储层温度和δ7Li值之间的相关性方程,即δ7Li=(-0.043±0.003)T+(11.9±0.5).这些结果表明地热水的Li同位素组成可以作为地质温度计.Millot等[38]完成了海水与玄武岩在25~250 ℃范围的水岩反应实验,建立了Li同位素分馏值与温度之间的经验关系式,即Δsolution-solid=7 847/T-8.093.并用这个经验关系式计算了马提尼克岛和瓜德罗普岛火山岛弧区域地热体系的储层温度,这些温度结果与地热水溶质温度估算公式计算的一致,表明了Li同位素体系是一个用于地热储层温度计算的有利工具.Millot等[44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... [101]发现,相对B和Sr同位素变化范围,Li同位素值显示了更窄的波动,并且很少依赖于储层岩性特征.进一步利用地热储层温度估算公式计算出的温度与地热流体的δ7Li值投图,建立了储层温度和δ7Li值之间的相关性方程,即δ7Li=(-0.043±0.003)T+(11.9±0.5).这些结果表明地热水的Li同位素组成可以作为地质温度计.Millot等[38]完成了海水与玄武岩在25~250 ℃范围的水岩反应实验,建立了Li同位素分馏值与温度之间的经验关系式,即Δsolution-solid=7 847/T-8.093.并用这个经验关系式计算了马提尼克岛和瓜德罗普岛火山岛弧区域地热体系的储层温度,这些温度结果与地热水溶质温度估算公式计算的一致,表明了Li同位素体系是一个用于地热储层温度计算的有利工具.Millot等[44]利用这个经验公式(Δsolution-solid=7 847/T-8.093)计算了法国巴黎盆地三叠系砂岩地热储层的温度,与化学温度估算公式计算的结果一致.以上研究说明无论是在火成岩地热体系还是沉积岩地热体系,温度控制着地热流体的Li同位素组成,δ7Li值可以用作地热储层温度的计算. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... ,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... ,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... ,101]. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Geothermal waters from the Taupo Volcanic Zone, New Zealand: Li, B and Sr isotopes characterization
12
2012
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水的运移需要一定动能,而地下热能的变化可以驱动地热流体的流动与运移,特别是火山区以及汇聚大陆边缘地热水的运移更加频繁.与传统同位素相比,Li同位素可以作为有效的工具预测深部地热流体流动与地震活动的关系.Nishio等[105]调查了日本中部火山地区地热水的Li同位素,发现来自地震震中的地热水样品以低的δ7Li值(-5.17‰~1.55‰)为特征,显示了下地壳深部流体的Li同位素组成性质,表明了Li来自下地壳,而其他地震不活跃地区的地热水样品呈现了更高的δ7Li值,显示了地热水与上地壳岩石水岩反应所呈现的Li同位素组成,代表了上地壳Li的信息.因此,这些以低δ7Li值为特征的地热流体来自下地壳深部地震的活动.当然,地热水低的δ7Li值也可以解释为一定温度下流体与岩石间Li同位素扩散交换的结果[102,103,120].然而,Nishio等[46]利用日本Nankai增生楔泥火山中流体的Li同位素特征成功证明了深部流体流动和地震活动间的联系. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... [102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... ,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
The source of halogens in geothermal fluids from the Taupo Volcanic Zone, North Island, New Zealand
11
2014
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
... [103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水的运移需要一定动能,而地下热能的变化可以驱动地热流体的流动与运移,特别是火山区以及汇聚大陆边缘地热水的运移更加频繁.与传统同位素相比,Li同位素可以作为有效的工具预测深部地热流体流动与地震活动的关系.Nishio等[105]调查了日本中部火山地区地热水的Li同位素,发现来自地震震中的地热水样品以低的δ7Li值(-5.17‰~1.55‰)为特征,显示了下地壳深部流体的Li同位素组成性质,表明了Li来自下地壳,而其他地震不活跃地区的地热水样品呈现了更高的δ7Li值,显示了地热水与上地壳岩石水岩反应所呈现的Li同位素组成,代表了上地壳Li的信息.因此,这些以低δ7Li值为特征的地热流体来自下地壳深部地震的活动.当然,地热水低的δ7Li值也可以解释为一定温度下流体与岩石间Li同位素扩散交换的结果[102,103,120].然而,Nishio等[46]利用日本Nankai增生楔泥火山中流体的Li同位素特征成功证明了深部流体流动和地震活动间的联系. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Lithium isotope geochemistry of the Yellowstone hydrothermal system
3
2003
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
Lithium and strontium isotopic systematics of waters around Ontake volcano, Japan: Implications for deep-seated fluids and earthquake swarms
6
2010
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水的运移需要一定动能,而地下热能的变化可以驱动地热流体的流动与运移,特别是火山区以及汇聚大陆边缘地热水的运移更加频繁.与传统同位素相比,Li同位素可以作为有效的工具预测深部地热流体流动与地震活动的关系.Nishio等[105]调查了日本中部火山地区地热水的Li同位素,发现来自地震震中的地热水样品以低的δ7Li值(-5.17‰~1.55‰)为特征,显示了下地壳深部流体的Li同位素组成性质,表明了Li来自下地壳,而其他地震不活跃地区的地热水样品呈现了更高的δ7Li值,显示了地热水与上地壳岩石水岩反应所呈现的Li同位素组成,代表了上地壳Li的信息.因此,这些以低δ7Li值为特征的地热流体来自下地壳深部地震的活动.当然,地热水低的δ7Li值也可以解释为一定温度下流体与岩石间Li同位素扩散交换的结果[102,103,120].然而,Nishio等[46]利用日本Nankai增生楔泥火山中流体的Li同位素特征成功证明了深部流体流动和地震活动间的联系. ...
Lithium isotopic systematics of hydrothermal vent fluids at the Main Endeavour Field, Northern Juan de Fuca Ridge
4
2004
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 数据来源:海底热液来自参考文献[38,106,107];大陆地热水来自参考文献[18,38,100~103,105] ...
Lithium isotopes in hydrothermally altered basalts from Hengill (SW Iceland)
4
2015
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... Concentration of lithium and its isotopic composition in natural waters
Table 1| 水体类型 | 来源 | Li/(μg/L) | δ7Li/‰ | 参考文献 |
|---|
| 海水 | 太平洋 | 175 | +31.8±1.9 | [80] |
| 海水标样BCR-403 | 185 | +31.0±0.1 | [83] |
| 西印度马尾藻海 | 180 | +32.4±1.0 | [89] |
| 日本海 | | +29.6±0.2 | [90] |
| 河水 | 长江 | 0.02~0.65 | 7.6~28.1 | [33] |
| 亚马孙河 | 0.67 | 22.1 | [91] |
| 密西西比河 | 2.6 | 16.7 | [91] |
| 雨水 | 法国 | 0.0006~0.0420 | 3.2~95.6 | [92] |
| 青海大柴达木 | 1 | 29.4 | [93] |
| 美国夏威夷 | 0.075 | 14.3 | [63] |
| 雪水 | 青海大柴达木 | 8 | 13.3 | [93] |
| 阿尔卑斯山 | 0.005~0.007 | | [94] |
| 南极 | ≤0.05 | | [95] |
| 盐湖卤水 | 死海 | 13 600 | 34.4 | [23] |
| 青海大柴达木湖 | 117 000~227 000 | 22.2±1.5 | [93] |
| 青海柴达木盆地 | 720~408 830 | 9.21~40.66 | [96] |
| 加拿大地盾 | 40~4 350 | 33.2~41.8 | [41] |
| 南美 | 80 000~7 000 000 | 9.0~13.3 | [19,97] |
| 大陆地热水 | 青海大柴达木湖热泉 | 2 340~3 250 | 2.29~4.33 | [93,96] |
| 云南—西藏地热带 | 4 240~25 000 | | [97] |
| 湖北江陵凹陷 | 48 000~65 000 | | [98,99] |
| 美国Mono盆地热泉 | 292~1 490 | 3.0~17.1 | [100] |
| 法国中央高原 | 52~153 000 | -0.1~10.9 | [43,101] |
| 法属群岛 | 0.5~10 100.0 | 3.8~7.8 | [38] |
| 新西兰陶波火山带 | 200~32 500 | -3~2 | [102,103] |
| 美国黄石公园 | 270~6 500 | 1.0~6.5 | [104] |
| 日本御岳火山区 | 0.57~2 370.00 | -5.17~12.60 | [105] |
| 南美安第斯山脉 | 7 000~147 400 | 1.4~18.4 | [18] |
| 海底热液 | Lau扩张中心和Juan de Fuca海岭 | 100~10 000 | 5~11 | [106,107] |
<strong>4.2</strong> 地热流体中<strong>Li</strong>同位素组成从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 数据来源:海底热液来自参考文献[38,106,107];法国地热水来自参考文献[101];新西兰地热水来自文献[102,103];法属群岛地热水来自参考文献[38];美国Mono盆地地热水来自参考文献[100];南美安第斯山脉地热水来自参考文献[18];日本御岳火山地区地热水来自参考文献[105] ...
... 数据来源:海底热液来自参考文献[38,106,107];大陆地热水来自参考文献[18,38,100~103,105] ...
Major geochemical characteristics of geothermal brines from the Upper Rhine Graben granitic basement with constraints on temperature and circulation
6
2016
... Li是极易溶的碱金属元素,自然界中不同流体含有不同的Li元素含量(表1).雨水和河水中Li含量较低,前者常小于3 μg/L[92],后者变化较大(0.2~20.0 μg/L)[91].相对而言,海水具有相对稳定的Li含量(约185 μg/L).地下水中有较高的Li含量,但其变化范围较大.与海底热液Li含量(0.1~10.0 mg/L)[23,37,61,106,107]相比,大陆地热水具有更高的Li含量(0.2~150.0 mg/L)[43,44,101,102,103].大陆地热水中较高的Li浓度与高温条件下水岩反应有关[18,43,108]. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... [108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... [108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
The effect of hydrothermal spring weathering processes and primary productivity on lithium isotopes: Lake Myvatn, Iceland
3
2016
... 从图1中可以看出,大陆地热流体的δ7Li值变化较大.例如,法国中央高原地热水的δ7Li值为-0.1‰~10.9‰[43,101];法属马提尼克岛和瓜德罗普岛的地热水的δ7Li值为3.8‰~7.8‰[38];新西兰陶波火山带的地热水δ7Li值为-3‰~2‰[102,103]、冰岛米瓦登湖受高温(200~300 ℃)地热水补给的河水δ7Li值为17.2‰~20.8‰[109];美国Mono盆地热泉水δ7Li值为3.0‰~17.1‰[100];美国黄石公园地热泉水δ7Li值为1.0‰~6.5‰[104];日本中部地区御岳火山附近地热水的δ7Li值为-5.17‰~12.60‰[105];中国的高温地热带主要位于藏南、川西以及滇西地区[97],其中地热水的δ7Li值未见报道,仅见青海大柴达木湖热泉的δ7Li值为2.29‰~4.33‰[93,96].统计这些Li同位素数据(图2),可以发现大陆地热流体的δ7Li值主要集中于-1‰~11‰,而海底热液呈现了更加窄的变化范围(3‰~9‰). ...
... 地热流体Li元素浓度和δ7Li值的较大波动表明其物质来源的复杂性.地热流体δ7Li值呈现了不同流体的混合,例如海水[44]、大气降水[44]以及深部流体[104,105].然而,不同流体的混合并不能解释地热流体所有δ7Li值的波动,例如法国巴黎盆地[44]、新西兰陶波火山带[102,103]和冰岛米瓦登湖[109]等地热体系中的δ7Li值特征.地热流体δ7Li值与温度和水岩反应密切相关. ...
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
Distribution and exploitation of geothermal resources in China
1
1991
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
中国地热资源的分布及其开发利用
1
1991
... 此外,根据地热系统中热能的来源,可简单分为岩浆热源型和非岩浆热源型水热系统[110].中国典型的岩浆热源型系统所排泄的地热水来自藏南和滇西地热带,储层温度大于150 ℃,其Li含量为4.24~25.00 mg/L[97].国外较典型的火山地热带为新西兰陶波火山区,储层最高温度可达330 ℃,其Li含量为0.2~32.5 mg/L,δ7Li值为-3‰~2‰[102,103].典型的非岩浆热源型系统所排泄的地热水为美国Mono盆地热泉水,储层温度小于100 ℃,其Li含量为0.292~1.490 mg/L,δ7Li值为3.0‰~17.1‰[100].对比上述两种不同类型热源型地热系统的Li含量和δ7Li值可以发现,来自岩浆热源型水热系统的地热水含有较高浓度的Li,且具有较低并均一的δ7Li值,而非岩浆热源型系统所排泄的地热水具有相对较低的Li浓度和不均一的δ7Li值.这归因于岩浆热源型地热系统中,岩性较均一,在高温条件下岩石与流体间的Li同位素分馏值小,Li从具有相似δ7Li值的源岩中被淋滤出来,从而具有高浓度的Li和均一的δ7Li值[103];反之,非岩浆热源型系统地热水Li含量及其同位素呈现了相反的特征. ...
Oxygen isotopic composition of sulphates, part 3: Oxygen isotopic fractionation in the bisulfate ion-water system
1
1969
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
An empirical Na-K-Ca geothermometer for natural waters
1
1973
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Magnesium correction to the Na-K-Ca chemical geothermometer
1
1979
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Chemical geothermometers applied to formation waters, gulf of Mexico and California Basins
1
1982
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Isotopic and chemical compositions of Parbati Valley geothermal discharges, Northwest Himalaya, India
1
1983
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Geothermal solute equilibria.Derivation of Na-K-Mg-Ca geoindicators
1
1988
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Behaviour of major elements and some trace elements (Li, Rb, Cs, Fe, Mn, W, F) in deep hot waters from granitic areas
1
1990
... 在地热体系中,地热水的溶质含量(K、Na、Ca、Mg、Li等)和氧同位素可用于储层温度的估算[111,112,113,114,115,116,117],这是水化学在地热储库勘探中的重要应用之一.这些储层温度估算公式是基于经验、半经验或矿物与水的平衡反应实验所获得. ...
Heterogeneities and interconnections in groundwaters: Coupled B, Li and stable-isotope variations in a large aquifer system (Eocene Sand aquifer, Southwestern France)
2
2012
... 法国巴黎盆地三叠系砂岩储层地热水的δ7Li值为-0.1‰~10.9‰[4],远低于海水的δ7Li值(31.5‰),接近于当地的大气降水(9.2‰~22.9‰)[92].可见,海水和雨水的混合来源并不能解释地热水的Li同位素特征,地热水与碎屑岩间的水岩反应改变了δ7Li值,有来自碎屑储层岩石释放Li的贡献[44].Millot等[38]调查了马提尼克岛和瓜德罗普岛地区地热水的Li同位素组成,其δ7Li值为4‰~26‰,其中大陆地热水和泉水的δ7Li值为3.8‰~7.8‰.该地热系统δ7Li值较大的波动并不仅仅由于海水的加入所致,不同温度下水岩反应过程中储层岩石释放了Li,同时改变了δ7Li值.日本中部火山地区地震震中地热水的Li同位素组成为-5.17‰~1.55‰,显示下地壳的流体信息,表明有深部流体的补给[105].新西兰陶波火山带的地热水δ7Li值(-3‰~2‰)显示了相对均一的变化范围[102,103].如此相对均一且低的δ7Li值归因于Li从具有相同δ7Li值的岩石中淋滤而来.冰岛米瓦登湖高温(200~300 ℃)地热泉水显示了相对均一且较高的δ7Li值变化范围(17.2‰~20.8‰)[109],这与地热储层不同岩性岩石与地热水所发生的水岩反应过程有关.Négrel等[118]发现了法国南部始新世低温(20~50 ℃)砂岩储库地热水不均一的δ7Li值(6.5‰~28.6‰),其Li来源被解释为多源的,不仅有大气降水来源,还有各种基岩水岩反应过程中释放了Li. ...
... 地热水中大多数元素的浓度受储层温度和岩石风化后的矿物组合控制,同时由于大陆地热储库岩性的复杂性,仅仅一个同位素的示踪往往会导致流体成因不完全的解释.因此,多同位素(Li-B-Sr-U)的示踪有重要的意义,不仅可以提供更加全面的地热水物源信息,而且还可以用于评价储层温度和岩性对地热水中这些同位素行为的控制作用[43,44,101,102,108].在大陆地热体系中,多同位素应用最广泛的地区当属法国中央高原[43,101]和巴黎盆地[44]、欧洲西部的莱茵河地堑上部地区[108]以及新西兰陶波火山带[102,103].Sr同位素信息可以反映沉积岩、变质岩或火成岩的贡献,B同位素的调查可以进一步证明涉及这些岩石的水岩反应过程和揭示水体的来源[43,44,101,102,118].相对于Sr和B同位素而言,温度对地热流体Li同位素组成起到重要的控制作用,可以示踪不同温度下的水岩反应过程,特别是高温条件[38,101]. ...
Developments in the understanding and application of lithium isotopes in the earth and planetary sciences
2
2004
... 其次,南美和北美西部高原地区地下富Li卤水或热泉的Li同位素值(1.4‰~18.4‰)也落在了大陆地热体系Li同位素值(-1‰~17‰)[119]范围内,显示了一定温度范围内水岩反应所导致的Li同位素值特征,表明了地下富Li卤水中Li的富集与高温水岩反应具有密切的关系. ...
... 不同的地质构造背景可形成不同类型的地热系统,首先,各类型地热体系Li同位素组成背景及其时空分布特征是怎样的尚不清楚,其次,这些体系内地热流体循环运移过程中Li同位素行为也是不清楚的,制约着Li同位素在大陆地热体系中的应用研究.大陆地热储库岩石主要为碎屑岩、碳酸盐岩和火成岩等[53,119,131],由于储库岩石的复杂性,其主要问题是地热流体与岩石间水岩反应过程中主要矿物相溶解和次生矿物形成机制是不清楚的,且该过程中Li同位素分馏机制还无法得到清楚的解释.这就需要我们注重不同温度下的水岩反应实验研究,定量化不同温度下次生矿物形成或Li被吸附于矿物表面所导致的Li同位素分馏机制.因此,将来在该方面的工作研究对地热体系中Li 的成因与演化的解释具有重要的意义.同时,我们也应该关注储层岩石的矿物学研究,地球化学与矿物学的双重验证才能更好地揭示地质作用过程. ...
Experimental determination of the role of diffusion on Li isotope fractionation during basaltic glass weathering
1
2011
... 地热水的运移需要一定动能,而地下热能的变化可以驱动地热流体的流动与运移,特别是火山区以及汇聚大陆边缘地热水的运移更加频繁.与传统同位素相比,Li同位素可以作为有效的工具预测深部地热流体流动与地震活动的关系.Nishio等[105]调查了日本中部火山地区地热水的Li同位素,发现来自地震震中的地热水样品以低的δ7Li值(-5.17‰~1.55‰)为特征,显示了下地壳深部流体的Li同位素组成性质,表明了Li来自下地壳,而其他地震不活跃地区的地热水样品呈现了更高的δ7Li值,显示了地热水与上地壳岩石水岩反应所呈现的Li同位素组成,代表了上地壳Li的信息.因此,这些以低δ7Li值为特征的地热流体来自下地壳深部地震的活动.当然,地热水低的δ7Li值也可以解释为一定温度下流体与岩石间Li同位素扩散交换的结果[102,103,120].然而,Nishio等[46]利用日本Nankai增生楔泥火山中流体的Li同位素特征成功证明了深部流体流动和地震活动间的联系. ...
Radiocarbon dating of groundwater of the aquifer confined in the Lower Triassic sandstones of the Lorraine region, France
1
1981
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
Radioelements, radiogenic helium and age relationships for groundwaters from the granites at Stripa, Sweden
1
1982
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
The chemistry and origin of groundwater in Triassic sandstone and Quaternary deposits, northwest England and some UK comparisons
1
1995
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
A multi-isotope (B, Sr, O, H, and C) and age dating (3H-3He and 14C) study of groundwater from Salinas Valley, California: Hydrochemistry, dynamics, and contamination process
1
2002
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
Relationship between surface water and groundwater in the Liujiang Basin-Hydrochemical constrains
1
2017
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
柳江盆地地表水与地下水转化关系的氢氧稳定同位素和水化学证据
1
2017
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
The theory and uses of natural uranium isotopic variations in Hydrology
1
1976
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
Uranium-series radionuclides in fluids and olids from the Milk River aquifer, Alberta, Canada
2
1991
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... [127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
U isotope systematics of groundwaters from the Triassic aquifer of the northeastern Paris Basin and of the Rhine Graben, France
2
2015
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
... [128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
Noble gases in crude oils from the Paris Basin, France: Implications for the origin of fluids and constraints on oil-water-gas interactions
1
1995
... 地热体系中Li同位素行为往往被解释为一定温度下流体与岩石所发生的水岩反应,而与水岩反应条件有关的流体停留时间等,尚未得到较好的约束.在评价地下水停留时间时,氢(3H)、碳(14C)和氦(4He)同位素常常被用到[121,122,123,124,125].相比而言,由于水体与沉积物之间234U和238U的放射性不平衡[126],即铀(U)系不平衡原理,使得U同位素成为评价地下水混合、循环时间和流速的有效工具之一[44,108,127,128].基于地下水的U同位素特征,Ivanovich等[127]建立了2种估算水体流速的模式:一种是在U含量稳定条件下,根据234U过剩衰变方程得出地下水的年龄,从而计算出水体的流速;另一种是在水体运输方程的基础上,考虑了放射性衰变和流动过程中发生的吸附作用2种因素的影响,从而得出水体的流速.其中,第二种模式计算出的流速与水力学模型计算出的结果一致.根据距离补给区不同位置地热水的234U/238U活度比,Millot等[44]得出了法国巴黎盆地三叠系地层流体循环的平均表观速度0.2 m/a,进而算出水体的停留时间约1.25 Ma,并与原油中稀有气体同位素估算的水体停留时间[129]一致.基于地下热液流体的U含量和活度比,Innocent等[128]进一步研究法国巴黎盆地东北部地下热水的流速,仅约为0.05 m/a.Sanjuan等[108]报道了莱茵河地堑上部地区地热水的U含量和活度比以及钍(Th)同位素特征,估算出深部地热卤水的最小运输时间约为1 000年.这些均说明了U同位素在估算地下流体流速和停留时间方面已经得到很好的应用,而热液体系中流体停留时间的确定对流体与岩石间水岩反应过程的理解具有重要的意义. ...
The tempo-spatial characteristics and forming mechanism of lithium-rich brines in China
1
2018
... 富Li卤水形成于干旱气候条件下的封闭盐湖盆地,同时在盆地内或周缘伴有火山或地热活动[18,97,130].水体中Li同位素值(δ7Li)已成为富Li卤水成因研究的有效工具.通过Li同位素行为的研究,表明地热流体中Li的富集与高温条件下的水岩反应有关[43,44,101,102,103,108].然而,温度对Li同位素的控制以及水岩反应过程中矿物相的变化更需要室内模拟实验的研究.Araoka等[17]对美国内华达州干盐湖中各种湖相沉积物开展了常温的淋滤实验研究,结果表明沉积物淋滤液的δ7Li值比河水和地下水对应的值低得多,接近火山岩的δ7Li值,并位于大陆热液流体的δ7Li值范围内,证明了Li主要通过热液活动中发生的水岩反应提供.但是这次淋滤实验并未能揭示温度以及主要矿物相溶解和次生矿物形成对Li同位素行为的控制.因此,不同温度下(25~300 ℃)水岩反应实验,例如水(盐水)与湖相沉积物、水(盐水)与玄武岩、水(盐水)与花岗岩,并结合矿物学的研究,更易于揭示和理解大陆地热体系中的Li同位素行为. ...
Research progress of the fractured-vuggy reservoir zones in carbonate reservoir
1
2018
... 不同的地质构造背景可形成不同类型的地热系统,首先,各类型地热体系Li同位素组成背景及其时空分布特征是怎样的尚不清楚,其次,这些体系内地热流体循环运移过程中Li同位素行为也是不清楚的,制约着Li同位素在大陆地热体系中的应用研究.大陆地热储库岩石主要为碎屑岩、碳酸盐岩和火成岩等[53,119,131],由于储库岩石的复杂性,其主要问题是地热流体与岩石间水岩反应过程中主要矿物相溶解和次生矿物形成机制是不清楚的,且该过程中Li同位素分馏机制还无法得到清楚的解释.这就需要我们注重不同温度下的水岩反应实验研究,定量化不同温度下次生矿物形成或Li被吸附于矿物表面所导致的Li同位素分馏机制.因此,将来在该方面的工作研究对地热体系中Li 的成因与演化的解释具有重要的意义.同时,我们也应该关注储层岩石的矿物学研究,地球化学与矿物学的双重验证才能更好地揭示地质作用过程. ...
碳酸盐岩储层缝洞储集体研究进展
1
2018
... 不同的地质构造背景可形成不同类型的地热系统,首先,各类型地热体系Li同位素组成背景及其时空分布特征是怎样的尚不清楚,其次,这些体系内地热流体循环运移过程中Li同位素行为也是不清楚的,制约着Li同位素在大陆地热体系中的应用研究.大陆地热储库岩石主要为碎屑岩、碳酸盐岩和火成岩等[53,119,131],由于储库岩石的复杂性,其主要问题是地热流体与岩石间水岩反应过程中主要矿物相溶解和次生矿物形成机制是不清楚的,且该过程中Li同位素分馏机制还无法得到清楚的解释.这就需要我们注重不同温度下的水岩反应实验研究,定量化不同温度下次生矿物形成或Li被吸附于矿物表面所导致的Li同位素分馏机制.因此,将来在该方面的工作研究对地热体系中Li 的成因与演化的解释具有重要的意义.同时,我们也应该关注储层岩石的矿物学研究,地球化学与矿物学的双重验证才能更好地揭示地质作用过程. ...




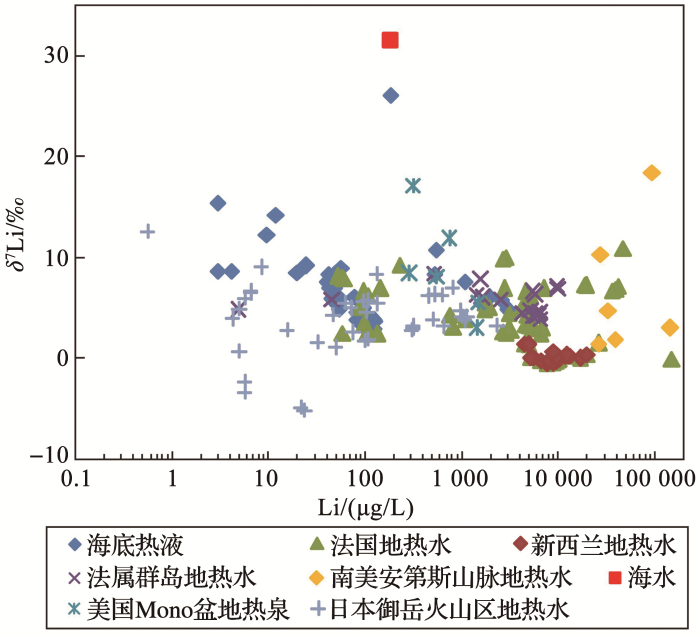
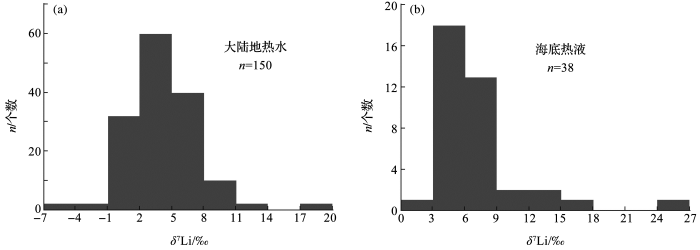


 甘公网安备62010202000687
甘公网安备62010202000687