

Advances on Soil Organic Carbon Dynamics Mediated by Microorganisms
Received date: 2023-08-02
Revised date: 2023-09-20
Online published: 2023-11-20
Supported by
the National Natural Science Foundation of China(41771216);The Guangzhou Science and Technology Plan Project(202201011738)
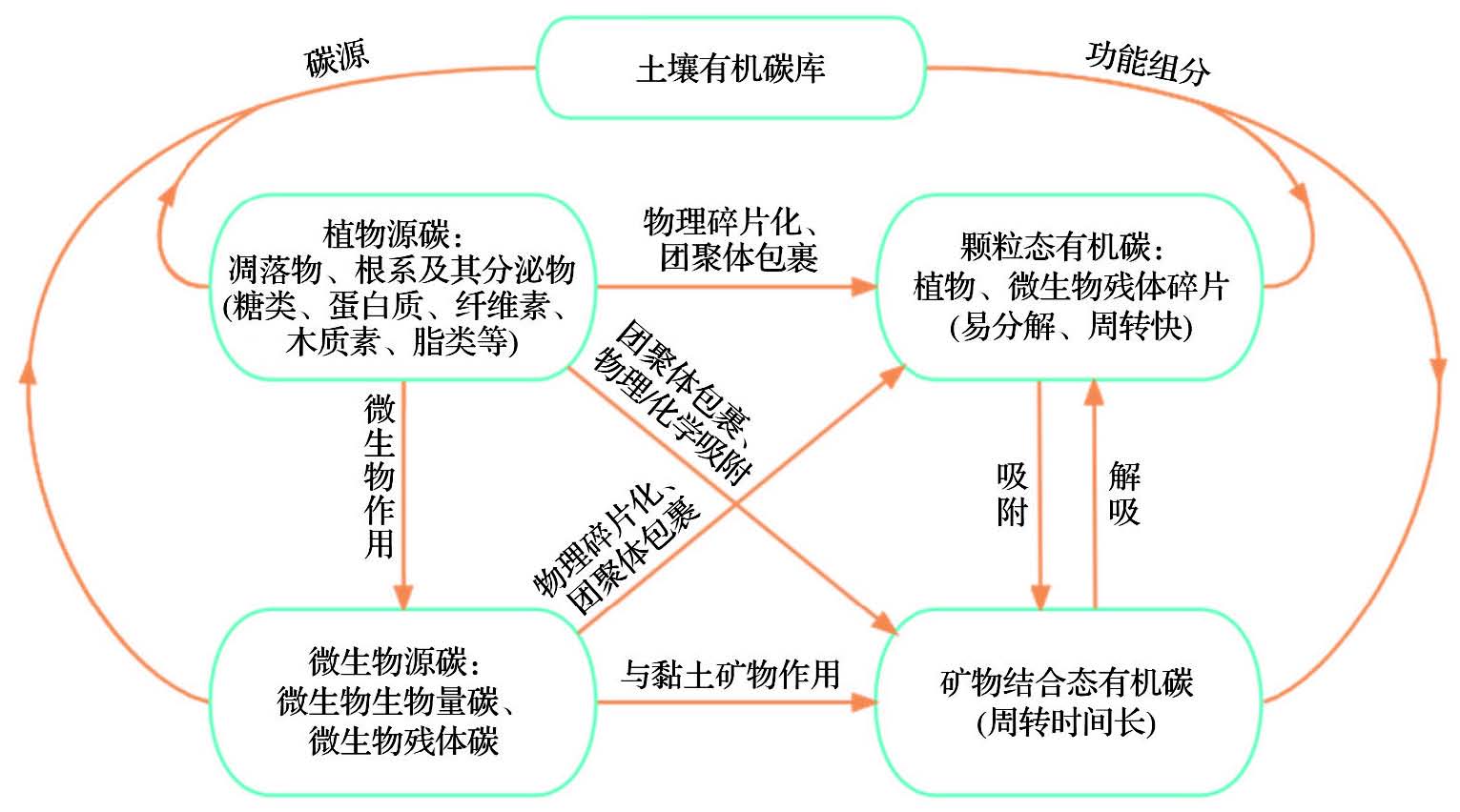
Soil Organic Carbon (SOC) is a crucial component of the carbon pool in terrestrial ecosystems because of its high storage capacity and long residence time. Slight changes in the SOC pool have a remarkable impact on terrestrial carbon flux and global climate change. The mechanisms of composition, transformation, and stability of SOC are primarily controlled by soil microbial properties. Therefore, the research results on the formation, transformation, and stabilization of SOC mediated by microorganisms are reviewed here, with the aim to further understand the function of soil carbon sequestration. SOC consists of plant- and microorganisms-driven carbons. Plant carbon is the major source of SOC. Soil microbial activity is the primary driving force of SOC formation, transformation, and stabilization. Soil microorganisms decompose plant carbon to form easy turnover soil particulate organic carbon through the “ex vivo modification” pathway. Microbial Residual Carbon (MRC) produced by soil microorganisms through the “in vivo turnover” pathway and Mineral-Associated Organic Carbon (MAOC) formed by the interaction with soil clay minerals contribute to stable SOC components, of which the contribution rate of MRC to stable SOC has been reported to be 38.74%. The equilibrium between the “priming and ongoing burial effects” regulates the storage and stability of SOC. On a global scale, the microbial activity mediating SOC changes is subject to annual precipitation and soil environmental factors (SOC, TN and pH). In response to global changes, the mechanism of SOC quantity and quality control by coupling plant litter, microbial activity, and soil matrix should be given more attention. In addition, environmental dependence of microbial carbon use efficiency should be focused to improve our understanding of the carbon sequestration effect of soil microorganisms.
Wenjie SONG , Yuzheng LIANG , Zhen TAO , Qingxiang ZHONG , Yicong HE . Advances on Soil Organic Carbon Dynamics Mediated by Microorganisms[J]. Advances in Earth Science, 2023 , 38(12) : 1213 -1223 . DOI: 10.11867/j.issn.1001-8166.2023.076
| 1 | CAMENZIND T, MASON-JONES K, MANSOUR I, et al. Formation of necromass-derived soil organic carbon determined by microbial death pathways[J]. Nature Geoscience, 2023, 16(2): 115-122. |
| 2 | WITZGALL K, VIDAL A, SCHUBERT D I, et al. Particulate organic matter as a functional soil component for persistent soil organic carbon[J]. Nature Communications, 2021, 12(1). DOI:10.1038/s41467-021-24192-8 . |
| 3 | KELL D B. Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: why and how[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2012, 367(1 595): 1 589-1 597. |
| 4 | LAL R. Soil carbon sequestration impacts on global climate change and food security[J]. Science, 2004, 304(5 677): 1 623-1 627. |
| 5 | HICKS PRIES C E, CASTANHA C, PORRAS R C, et al. The whole-soil carbon flux in response to warming[J]. Science, 2017, 355(6 332): 1 420-1 423. |
| 6 | LEHMANN J, KLEBER M. The contentious nature of soil organic matter[J]. Nature, 2015, 528(7 580): 60-68. |
| 7 | RASSE D P, RUMPEL C, DIGNAC M F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation[J].Plant and Soil, 2005, 269(1/2): 341-356. |
| 8 | KUZYAKOV Y, FRIEDEL J K, STAHR K. Review of mechanisms and quantification of priming effects[J]. Soil Biology and Biochemistry, 2000, 32(11/12): 1 485-1 498. |
| 9 | SCHIMEL J P, SCHAEFFER S M. Microbial control over carbon cycling in soil[J]. Frontiers in Microbiology, 2012, 3. DOI: 10.3389/fmicb.2012.00348 . |
| 10 | LIANG C, SCHIMEL J P, JASTROW J D. The importance of anabolism in microbial control over soil carbon storage[J]. Nature Microbiology, 2017, 2(8): 1-6. |
| 11 | JOOS F, PRENTICE I C, SITCH S, et al. Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) emission scenarios[J]. Global Biogeochemical Cycles, 2001, 15(4): 891-907. |
| 12 | PATERSON E, HALL J M, RATTRAY E A S, et al. Effect of elevated CO2 on rhizosphere carbon flow and soil microbial processes[J]. Global Change Biology, 1997, 3(4): 363-377. |
| 13 | KUZYAKOV Y. Priming effects: interactions between living and dead organic matter[J]. Soil Biology and Biochemistry, 2010, 42(9): 1 363-1 371. |
| 14 | KEILUWEIT M, BOUGOURE J J, NICO P S, et al. Mineral protection of soil carbon counteracted by root exudates[J]. Nature Climate Change, 2015, 5(6): 588-595. |
| 15 | VORONEY R P, PAUL E A, ANDERSON D W. Decomposition of wheat straw and stabilization of microbial products[J]. Canadian Journal of Soil Science, 1989, 69(1): 63-77. |
| 16 | MARTIN J P, HAIDFR K, FARMKR W J, et al. Decomposition and distribution of residual activity of some 13C-microbial polysaccharides and cells, glucose, cellulose and wheat straw in soil[J]. Soil Biology and Biochemistry, 1974, 6(4): 221-230. |
| 17 | SOONG J L, COTRUFO M F. Annual burning of a tallgrass prairie inhibits C and N cycling in soil, increasing recalcitrant pyrogenic organic matter storage while reducing N availability[J]. Global Change Biology, 2015, 21(6): 2 321-2 333. |
| 18 | COTRUFO M F, SOONG J L, HORTON A J, et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss[J]. Nature Geoscience, 2015, 8(10): 776-779. |
| 19 | KAISER K, KALBITZ K. Cycling downwards-dissolved organic matter in soils[J]. Soil Biology and Biochemistry, 2012, 52: 29-32. |
| 20 | COTRUFO M F, WALLENSTEIN M D, BOOT C M, et al. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter?[J]. Global Change Biology, 2013, 19(4): 988-995. |
| 21 | MAMBELLI S, BIRD J A, GLEIXNER G, et al. Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation[J]. Organic Geochemistry, 2011, 42(9): 1 099-1 108. |
| 22 | SOKOL N W, BRADFORD M A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input[J]. Nature Geoscience, 2019, 12(1): 46-53. |
| 23 | SOKOL N, KUEBBING S E, KARLSEN-AYALA E, et al. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon[J]. New Phytologist, 2018. DOI:10.1111/nph.15361 . |
| 24 | OTTO A, SHUNTHIRASINGHAM C, SIMPSON M J. A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada[J]. Organic Geochemistry, 2005, 36(3): 425-448. |
| 25 | LIANG C, AMELUNG W, LEHMANN J, et al. Quantitative assessment of microbial necromass contribution to soil organic matter[J]. Global Change Biology, 2019, 25(11): 3 578-3 590. |
| 26 | NI X Y, LIAO S, TAN S Y, et al. A quantitative assessment of amino sugars in soil profiles[J]. Soil Biology and Biochemistry, 2020, 143. DOI:10.1016/j.soilbio.2020.107762 . |
| 27 | ZHANG X D, AMELUNG W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils[J]. Soil Biology and Biochemistry, 1996, 28(9): 1 201-1 206. |
| 28 | PATOINE G, EISENHAUER N, CESARZ S, et al. Drivers and trends of global soil microbial carbon over two decades[J]. Nature Communications, 2022, 13. DOI:10.1038/s41467-022-31833-z . |
| 29 | LAVALLEE J M, SOONG J L, COTRUFO M F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century[J]. Global Change Biology, 2020, 26(1): 261-273. |
| 30 | YANG Ying, WU Fuzhong, WU Qiuxia, et al. Soil organic carbon associated with iron oxides in terrestrial ecosystems: content, distribution and control[J]. Chinese Science Bulletin, 2023, 68(6): 695-704. |
| 30 | 杨颖, 吴福忠, 吴秋霞, 等. 陆地生态系统土壤铁结合态有机碳: 含量、分布与调控[J]. 科学通报, 2023, 68(6): 695-704. |
| 31 | XIAO K Q, ZHAO Y, LIANG C, et al. Introducing the soil mineral carbon pump[J]. Nature Reviews Earth & Environment, 2023, 4(3): 135-136. |
| 32 | COTRUFO M F, LAVALLEE J M. Soil organic matter formation, persistence, and functioning: a synthesis of current understanding to inform its conservation and regeneration[M]// Advances in agronomy. Amsterdam: Elsevier, 2022: 1-66. |
| 33 | HERNDON E M, MARTíNEZ C E, BRANTLEY S L. Spectroscopic (XANES/XRF) characterization of contaminant Manganese cycling in a temperate watershed[J]. Biogeochemistry, 2014, 121(3): 505-517. |
| 34 | KRAMER M G, CHADWICK O A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale[J]. Nature Climate Change, 2018, 8(12): 1 104-1 108. |
| 35 | MAN M L, PIERSON D, CHIU R, et al. Twenty years of litter manipulation reveals that above-ground litter quantity and quality controls soil organic matter molecular composition[J]. Biogeochemistry, 2022, 159(3): 393-411. |
| 36 | ALMEIDA L F J, SOUZA I F, HURTARTE L C C, et al. Forest litter constraints on the pathways controlling soil organic matter formation[J]. Soil Biology and Biochemistry, 2021, 163. DOI:10.1016/j.soilbio.2021.108447 . |
| 37 | CANESSA R, BRINK L, SALDA?A A, et al. Relative effects of climate and litter traits on decomposition change with time, climate and trait variability[J]. Journal of Ecology, 2020, 109: 447-458. |
| 38 | CASTELLANO M J, MUELLER K E, OLK D C, et al. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept[J]. Global Change Biology, 2015, 21(9): 3 200-3 209. |
| 39 | WONG D W S. Structure and action mechanism of ligninolytic enzymes[J]. Applied Biochemistry and Biotechnology, 2009, 157(2): 174-209. |
| 40 | KHAN M U, AHRING B K. Lignin degradation under anaerobic digestion: influence of lignin modifications—a review[J]. Biomass and Bioenergy, 2019, 128. DOI:10.1016/J.BIOMBIOE.2019.105325 . |
| 41 | VALá?KOVá V, BALDRIAN P. Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus-production of extracellular enzymes and characterization of the major cellulases[J]. Microbiology, 2006, 152(12): 3 613-3 622. |
| 42 | LI H Y, WANG H, WANG H T, et al. The chemodiversity of paddy soil dissolved organic matter correlates with microbial community at continental scales[J].Microbiome, 2018, 6(1): 1-16. |
| 43 | KALBITZ K, SCHWESIG D, SCHMERWITZ J, et al. Changes in properties of soil-derived dissolved organic matter induced by biodegradation[J]. Soil Biology and Biochemistry, 2003, 35(8): 1 129-1 142. |
| 44 | KLEBER M, SOLLINS P, SUTTON R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces[J]. Biogeochemistry, 2007, 85(1): 9-24. |
| 45 | BARDGETT R D, van der PUTTEN W H. Belowground biodiversity and ecosystem functioning[J]. Nature, 2014, 515(7 528): 505-511. |
| 46 | WANG B R, AN S S, LIANG C, et al. Microbial necromass as the source of soil organic carbon in global ecosystems[J]. Soil Biology and Biochemistry, 2021, 162. DOI:10.1016/J.BIOMBIOE.2019.105325 . |
| 47 | ENKHMAA E, WANG C W, YU W Y, et al. Carbon versus nitrogen release from root and leaf litter is modulated by litter position and plant functional type[J]. Journal of Ecology, 2022, 111(1): 198-213. |
| 48 | HE W, XU X, ZHANG C C, et al. Understory vegetation removal reduces the incidence of non-additive mass loss during leaf litter decomposition in a subtropical Pinus massoniana plantation[J]. Plant and Soil, 2020, 446(1): 529-541. |
| 49 | DU N N, LI W R, QIU L P, et al. Mass loss and nutrient release during the decomposition of sixteen types of plant litter with contrasting quality under three precipitation regimes[J]. Ecology and Evolution, 2020, 10(7): 3 367-3 382. |
| 50 | ESCH E H, KING J Y, CLELAND E E. Foliar litter chemistry mediates susceptibility to UV degradation in two dominant species from a semi-arid ecosystem[J]. Plant and Soil, 2019, 440(1): 265-276. |
| 51 | CRAIG M E, GEYER K M, BEIDLER K V, et al. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits[J]. Nature Communications, 2022, 13(1): 1-10. |
| 52 | SHAO P S, LYNCH L, XIE H T, et al. Tradeoffs among microbial life history strategies influence the fate of microbial residues in subtropical forest soils[J]. Soil Biology and Biochemistry, 2021, 153. DOI:10.1016/j.soilbio.2020.108112 . |
| 53 | BHOPLE P, KEIBLINGER K, DJUKIC I, et al. Microbial necromass formation, enzyme activities and community structure in two alpine elevation gradients with different bedrock types[J]. Geoderma, 2021, 386. DOI:10.1016/j.geoderma.2020.114922 . |
| 54 | PROMMER J, WALKER T W N, WANEK W, et al. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity[J]. Global Change Biology, 2020, 26(2): 669-681. |
| 55 | WINZLER R J, BAUMBERGER J P. The degradation of energy in the metabolism of yeast cells[J]. Journal of Cellular and Comparative Physiology, 1938, 12(2): 183-211. |
| 56 | SINSABAUGH R L, TURNER B L, TALBOT J M, et al. Stoichiometry of microbial carbon use efficiency in soils[J]. Ecological Monographs, 2016, 86(2): 172-189. |
| 57 | KALLENBACH C M, FREY S D, GRANDY A S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls[J]. Nature Communications, 2016, 7(1): 1-10. |
| 58 | SAIFUDDIN M, BHATNAGAR J M, SEGRè D, et al. Microbial carbon use efficiency predicted from genome-scale metabolic models[J]. Nature Communications, 2019, 10(1): 1-10. |
| 59 | PAUSCH J, KUZYAKOV Y. Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale[J]. Global Change Biology, 2018, 24(1): 1-12. |
| 60 | K?STNER M, MILTNER A, THIELE-BRUHN S, et al. Microbial necromass in soils—linking microbes to soil processes and carbon turnover[J]. Frontiers in Environmental Science, 2021, 9. DOI:10.3389/fenvs.2021.756378 . |
| 61 | SAADAT N, NIES T, ROUSSET Y, et al. Thermodynamic limits and optimality of microbial growth[J]. Entropy, 2020, 22(3). DOI: 10.3390/e22030277 . |
| 62 | LU J Y, YANG J F, KEITEL C, et al. Rhizosphere priming effects of Lolium perenne and Trifolium repens depend on phosphorus fertilization and biological nitrogen fixation[J]. Soil Biology and Biochemistry, 2020, 150. DOI:10.1016/j.soilbio.2020.108005 . |
| 63 | SCHIMEL D S. Terrestrial ecosystems and the carbon cycle[J]. Global Change Biology, 1995, 1. DOI:10.1111/j.1365-2486.1995.tb00008.x . |
| 64 | YIN L M, ZHANG T S, DIJKSTRA F A, et al. Priming effect varies with root order: a case of Cunninghamia lanceolata [J]. Soil Biology and Biochemistry, 2021, 160. DOI:10.1016/j.soilbio.2021.108354 . |
| 65 | LIANG J Y, ZHOU Z H, HUO C F, et al. More replenishment than priming loss of soil organic carbon with additional carbon input[J]. Nature Communications, 2018, 9(1): 1-9. |
| 66 | LIU X, XIONG Y M, LIAO B W. Relative contributions of leaf litter and fine roots to soil organic matter accumulation in mangrove forests[J]. Plant and Soil, 2017, 421(1): 493-503. |
| 67 | GEETHANJALI P A, JAYASHANKAR P M. A review on litter decomposition by soil fungal community[J]. IOSR Journal of Pharmacy and Biological Sciences, 2016, 11(4): 1-3. |
| 68 | WILHELM R C, SINGH R, ELTIS L D, et al. Bacterial contributions to delignification and lignocellulose degradation in forest soils with metagenomic and quantitative stable isotope probing[J]. The ISME Journal, 2019, 13(2): 413-429. |
| 69 | SINSABAUGH R L, LAUBER C L, WEINTRAUB M N, et al. Stoichiometry of soil enzyme activity at global scale[J]. Ecology Letters, 2008, 11(11): 1 252-1 264. |
| 70 | MORRISSEY E M, BERRIER D J, NEUBAUER S C, et al. Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland[J]. Biogeochemistry, 2014, 117(2): 473-490. |
| 71 | PEI L X, YE S Y, YUAN H M, et al. Glomalin-related soil protein distributions in the wetlands of the Liaohe Delta, Northeast China: implications for carbon sequestration and mineral weathering of coastal wetlands[J]. Limnology and Oceanography, 2020, 65(5): 979-991. |
| 72 | RASANAYAGAM S, JEFFRIES P. Production of acid is responsible for antibiosis by some ectomycorrhizal fungi[J]. Mycological Research, 1992, 96(11): 971-976. |
| 73 | ORWIN K H, KIRSCHBAUM M U F, St JOHN M G, et al. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment[J]. Ecology Letters, 2011, 14(5): 493-502. |
| 74 | AVERILL C, TURNER B L, FINZI A C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage[J]. Nature, 2014, 505(7 484): 543-545. |
| 75 | SULMAN B N, BRZOSTEK E R, MEDICI C, et al. Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association[J]. Ecology Letters, 2017, 20(8): 1 043-1 053. |
| 76 | FERNANDEZ C W, KOIDE R T. Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter[J]. Soil Biology and Biochemistry, 2014, 77: 150-157. |
| 77 | FREY S D, SIX J, ELLIOTT E T. Reciprocal transfer of carbon and nitrogen by decomposer fungi at the soil-litter interface[J]. Soil Biology and Biochemistry, 2003, 35(7): 1 001-1 004. |
| 78 | FERNANDEZ C W, LANGLEY J A, CHAPMAN S, et al. The decomposition of ectomycorrhizal fungal necromass[J]. Soil Biology and Biochemistry, 2016, 93: 38-49. |
| 79 | TISDALL J M, OADES J M. Organic matter and water-stable aggregates in soils[J]. Journal of Soil Science, 1982, 33(2): 141-163. |
| 80 | CHENG L, ZHANG N F, YUAN M T, et al. Warming enhances old organic carbon decomposition through altering functional microbial communities[J]. The ISME Journal, 2017, 11(8): 1 825-1 835. |
| 81 | SOONG J L, FUCHSLUEGER L, MARA?ON-JIMENEZ S, et al. Microbial carbon limitation: the need for integrating microorganisms into our understanding of ecosystem carbon cycling[J]. Global Change Biology, 2020, 26(4): 1 953-1 961. |
| 82 | TIAN J, ZONG N, HARTLEY I P, et al. Microbial metabolic response to winter warming stabilizes soil carbon[J]. Global Change Biology, 2021, 27(10): 2 011-2 028. |
| 83 | BAI T S, WANG P, HALL S J, et al. Interactive global change factors mitigate soil aggregation and carbon change in a semi-arid grassland[J]. Global Change Biology, 2020, 26(9): 5 320-5 332. |
| 84 | XIA J Y, WAN S Q. Global response patterns of terrestrial plant species to nitrogen addition[J]. The New Phytologist, 2008, 179(2): 428-439. |
| 85 | YE C L, CHEN D M, HALL S J, et al. Reconciling multiple impacts of nitrogen enrichment on soil carbon: plant, microbial and geochemical controls[J]. Ecology Letters, 2018, 21(8): 1 162-1 173. |
| 86 | SCHIMEL J P, GULLEDGE J M, CLEIN-CURLEY J S, et al. Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga[J]. Soil Biology and Biochemistry, 1999, 31(6): 831-838. |
| 87 | SANTONJA M, FERNANDEZ C, GAUQUELIN T, et al. Climate change effects on litter decomposition: intensive drought leads to a strong decrease of litter mixture interactions[J]. Plant and Soil, 2015, 393(1): 69-82. |
| 88 | HEMKEMEYER M, CHRISTENSEN B T, MARTENS R, et al. Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants[J]. Soil Biology and Biochemistry, 2015, 90: 255-265. |
| 89 | MIN K, LEHMEIER C A, IV F B, et al. Carbon availability modifies temperature responses of heterotrophic microbial respiration, carbon uptake affinity, and stable carbon isotope discrimination[J]. Frontiers in Microbiology, 2016, 7. DOI:10.3389/fmicb.2016.02083 . |
| 90 | PENG X Q, WANG W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of Northern China[J]. Soil Biology and Biochemistry, 2016, 98: 74-84. |
| 91 | JONES D L, COOLEDGE E C, HOYLE F C, et al. pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities[J]. Soil Biology and Biochemistry, 2019, 138. DOI:10.1016/j.soilbio.2019.107584 . |
| 92 | TAO F, HUANG Y Y, HUNGATE B A, et al. Microbial carbon use efficiency promotes global soil carbon storage[J]. Nature, 2023, 618(7 967): 981-985. |
/
| 〈 |
|
〉 |