

溶解性黑碳特征结构、环境行为与生态效应研究进展
收稿日期: 2025-06-06
修回日期: 2025-07-02
网络出版日期: 2025-07-03
基金资助
黄淮实验室科创专项项目(240700001)
Progress on the Characteristic Structure, Environmental Behaviors, and Ecological Effects of Dissolved Black Carbon
Received date: 2025-06-06
Revised date: 2025-07-02
Online published: 2025-07-03
Supported by
the Huanghuai Laboratory Science & Technology Innovation Project(240700001)
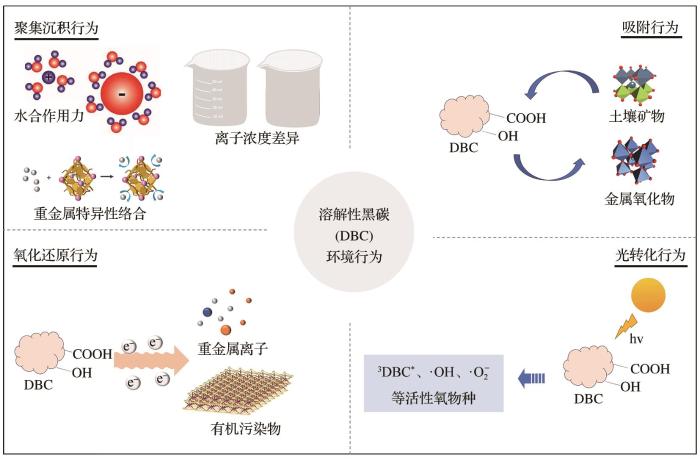
溶解性黑碳作为黑碳连续体的关键活性组分,其分子特征结构和环境归趋显著区别于颗粒态黑碳。溶解性黑碳可通过界面络合、氧化还原调控及生物代谢等重要途径,深度参与元素及化合物的生物地球化学循环过程。通过系统解析溶解性黑碳的结构异质性,聚焦其在多介质界面的团聚、转化和吸附等行为,阐明其通过调控元素循环(碳封存、氮还原)、矿物转化、污染物迁移转化及微生物/植物代谢等途径,深度扰动生态系统土壤团聚体与生物群落的结构,进而影响生态系统的碳汇能力和生物多样性。未来研究需突破溶解性黑碳分子指纹图谱的异构体解析、界面反应动力学原位定量及微界面动态表征等技术瓶颈,揭示溶解性黑碳—元素(污染物)耦合体系的演变规律与生态风险,为精准评估黑碳循环的生态环境风险提供理论支撑。
鄂正阳 , 丁哲 , 梅徽阳 , 张伟超 , 李海松 , 梁建军 , 李平 , 范桥辉 . 溶解性黑碳特征结构、环境行为与生态效应研究进展[J]. 地球科学进展, 2025 , 40(7) : 725 -736 . DOI: 10.11867/j.issn.1001-8166.2025.053
As a key active component of the black carbon continuum, Dissolved Black Carbon (DBC) exhibits markedly distinct molecular structural characteristics and environmental fate attributes compared with particulate black carbon. Originating from the incomplete combustion of biomass and fossil fuels, DBC is highly reactive and mobile, with colloidal particles facilitating its transport approximately three times faster than that of particulate black carbon. This enables extensive participation in biogeochemical cycles through interfacial complexation, redox regulation, and biological metabolism. These processes are integral to the Earth’s material cycles and energy transformations. This study systematically analyzed the structural heterogeneity of DBC derived from various sources, emphasizing its environmental behavior, such as aggregation influenced by cation valency and salinity, adsorption onto mineral surfaces, redox-mediated transformation of heavy metals, and photochemical reactions across soil-water-atmosphere interfaces. We further elucidated how DBC profoundly influences ecosystem structure and function by regulating elemental cycles (e.g., enhancing carbon sequestration and promoting nitrate reduction), mediating iron mineral transformation, facilitating contaminant transport and transformation, and exerting dual effects on microbial and plant metabolism. Its complex role is evident as it can serve as a nutrient source yet also induce oxidative stress or enhance heavy metal uptake in crops. However, current understanding is constrained by technical limitations in resolving molecular fingerprint isomers, quantifying interfacial reaction kinetics in situ, and dynamically characterizing micro interfacial processes. Overcoming these bottlenecks is essential to unravel the evolutionary mechanisms, interface dynamics, and ecological risks of DBC-pollutant/element coupling systems. This review synthesizes the current knowledge and aims to provide a theoretical foundation for accurately assessing the ecological and environmental impacts of black carbon cycling in the context of global change. This further highlights the need for advanced predictive models and in-situ techniques to support ecological conservation, pollution control, and sustainable environmental management.

| [1] | JAFFé R, DING Y, NIGGEMANN J, et al. Global charcoal mobilization from soils via dissolution and riverine transport to the oceans[J]. Science, 2013, 340(6 130): 345-347. |
| [2] | LU Y, CAI Y W, ZHANG S, et al. Application of biochar-based photocatalysts for adsorption-(photo)degradation/reduction of environmental contaminants: mechanism, challenges and perspective[J]. Biochar, 2022, 4(1). DOI: 10.1007/s42773-022-00173-y . |
| [3] | XU Qian, XIAO Cunde, FENG Yaru, et al. Advances in microbe mediated key processes of the carbon cycle in thermokarst lakes[J]. Advances in Earth Science, 2023, 38(5): 470-482. |
| 许茜, 效存德, 冯雅茹, 等. 微生物介导的热融湖碳循环关键过程研究进展[J]. 地球科学进展,2023, 38(5): 470-482. | |
| [4] | CHANG Z F, TIAN L P, LI F F, et al. Organo-mineral complexes protect condensed organic matter as revealed by benzene-polycarboxylic acids[J]. Environmental Pollution, 2020, 260. DOI: 10.1016/j.envpol.2020.113977 . |
| [5] | SANTíN C, DOERR S H, MERINO A, et al. Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars[J]. Scientific Reports, 2017, 7(1). DOI: 10.1038/s41598-017-10455-2 . |
| [6] | WARD C P, SLEIGHTER R L, HATCHER P G, et al. Insights into the complete and partial photooxidation of black carbon in surface waters[J]. Environmental Science Processes & Impacts, 2014, 16(4): 721-731. |
| [7] | SUN C, CHEN T, HUANG Q X, et al. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: performance and mechanism[J]. Chemical Engineering Journal, 2020, 380. DOI: 10.1016/j.cej.2019.122519 . |
| [8] | ZHOU L, ZHOU Y Q, TANG X M, et al. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter[J]. Water Research, 2021, 190. DOI: 10.1016/j.watres.2020.116776 . |
| [9] | ZHU Tangliu, LIU Zhidan, ZOU Li, et al. Spectral characterization of chromophoric dissolved organic matter in the interstitial water of land-sea interaction strata along the south coast of Laizhou Bay[J]. Advances in Earth Science, 2024, 39(6): 647-658. |
| 祝唐刘, 刘智丹, 邹立, 等. 莱州湾南岸陆海交互地层间隙水有色溶解有机物的光谱特征研究[J]. 地球科学进展, 2024, 39(6): 647-658. | |
| [10] | CHUN Y, SHENG G Y, CHIOU C T, et al. Compositions and sorptive properties of crop residue-derived chars[J]. Environmental Science & Technology, 2004, 38(17): 4 649-4 655. |
| [11] | ZHENGYANG E, LIANG J J, LI P, et al. A review on photocatalytic attribution and process of pyrolytic biochar in environment[J]. Water Research, 2024, 251. DOI: 10.1016/j.watres.2023.120994 . |
| [12] | COPPOLA A I, SEIDEL M, WARD N D, et al. Marked isotopic variability within and between the Amazon River and marine dissolved black carbon pools[J]. Nature Communications, 2019, 10(1). DOI: 10.1038/s41467-019-11543-9 . |
| [13] | LUO L, CHEN Z E, LV J T, et al. Molecular understanding of dissolved black carbon sorption in soil-water environment[J]. Water Research, 2019, 154: 210-216. |
| [14] | WAGNER S, JAFFé R, STUBBINS A. Dissolved black carbon in aquatic ecosystems[J]. Limnology and Oceanography Letters, 2018, 3(3): 168-185. |
| [15] | QU X L, FU H Y, MAO J D, et al. Chemical and structural properties of dissolved black carbon released from biochars[J]. Carbon, 2016, 96: 759-767. |
| [16] | FU H Y, LIU H T, MAO J D, et al. Photochemistry of dissolved black carbon released from biochar: reactive oxygen species generation and phototransformation[J]. Environmental Science & Technology, 2016, 50(3): 1 218-1 226. |
| [17] | YANG C H, LIU Y Z, SUN X M, et al. Characterization of fluorescent dissolved organic matter from green macroalgae (Ulva prolifera)-derived biochar by excitation-emission matrix combined with parallel factor and self-organizing maps analyses[J]. Bioresource Technology, 2019, 287. DOI: 10.1016/j.biortech.2019.121471 . |
| [18] | VALLE J, GONSIOR M, HARIR M, et al. Extensive processing of sediment pore water dissolved organic matter during anoxic incubation as observed by high-field mass spectrometry (FTICR-MS)[J]. Water Research, 2018, 129: 252-263. |
| [19] | CHEN L, DUAN J, DU P H, et al. Accurate identification of radicals by in situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes[J]. Water Research, 2022, 221. DOI: 10.1016/j.watres.2022.118747 . |
| [20] | WAGNER S, DING Y, JAFFé R. A new perspective on the apparent solubility of dissolved black carbon[J]. Frontiers in Earth Science, 2017, 5. DOI: 10.3389/feart.2017.00075 . |
| [21] | GRABER E R, TSECHANSKY L, LEW B, et al. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe[J]. European Journal of Soil Science, 2014, 65(1): 162-172. |
| [22] | QUALLS R G, HAINES B L. Geochemistry of dissolved organic nutrients in water percolating through a forest ecosystem[J]. Soil Science Society of America Journal, 1991, 55(4): 1 112-1 123. |
| [23] | LIU Y R, ZHU T T, REN S Q, et al. Contribution of nitrification and denitrification to nitrous oxide turnovers in Membrane-Aerated Biofilm Reactors (MABR): a model-based evaluation[J]. The Science of the Total Environment, 2022, 806(Pt 3). DOI: 10.1016/j.scitotenv.2021.151321 . |
| [24] | CHU Q N, XUE L H, WANG B Y, et al. Insights into the molecular transformation in the dissolved organic compounds of agro-waste-hydrochars by microbial-aging using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Bioresource Technology, 2021, 320. DOI: 10.1016/j.biortech.2020.124411 . |
| [25] | HAGEMANN N, JOSEPH S, SCHMIDT H P, et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility[J]. Nature Communications, 2017, 8(1). DOI: 10.1038/s41467-017-01123-0 . |
| [26] | OHNO T, PARR T B, GRUSELLE M C I, et al. Molecular composition and biodegradability of soil organic matter: a case study comparing two new England forest types[J]. Environmental Science & Technology, 2014, 48(13): 7 229-7 236. |
| [27] | JAMIESON T, SAGER E, GUéGUEN C. Characterization of biochar-derived dissolved organic matter using UV-visible absorption and excitation-emission fluorescence spectroscopies[J]. Chemosphere, 2014, 103: 197-204. |
| [28] | CANTRELL K B, HUNT P G, UCHIMIYA M, et al. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar[J]. Bioresource Technology, 2012, 107: 419-428. |
| [29] | XU F C, WEI C H, ZEN Q Q, et al. Aggregation behavior of dissolved black carbon: implications for vertical mass flux and fractionation in aquatic systems[J]. Environmental Science & Technology, 2017, 51 (23): 13 723-13 732. |
| [30] | XU Y H, OU Q, LIU C H, et al. Aggregation and deposition behaviors of dissolved black carbon with coexisting heavy metals in aquatic solution[J]. Environmental Science: Nano, 2020, 7 (9): 2 773-2 784. |
| [31] | TANG N, GUO Y H, ZHU Z Q, et al. New insights into aggregation behaviors of the UV-irradiated dissolved biochars (DBioCs) in aqueous environments: effects of water chemistries and variation in the hamaker constant[J]. Environmental Science & Technology, 2024, 58(18): 8 053-8 064. |
| [32] | AVNERI-KATZ S, YOUNG R B, MCKENNA A M, et al. Adsorptive fractionation of Dissolved Organic Matter (DOM) by mineral soil: macroscale approach and molecular insight[J]. Organic Geochemistry, 2017, 103: 113-124. |
| [33] | Lü J T, ZHANG S Z, WANG S S, et al. Molecular-scale investigation with ESI-FT-ICR-MS on fractionation of dissolved organic matter induced by adsorption on iron oxyhydroxides[J]. Environmental Science & Technology, 2016, 50(5): 2 328-2 336. |
| [34] | KAISER K, GUGGENBERGER G, HAUMAIER L, et al. Dissolved organic matter sorption on sub soils and minerals studied by 13C-NMR and DRIFT spectroscopy[J]. European Journal of Soil Science, 1997, 48(2): 301-310. |
| [35] | KOTHAWALA D N, ROEHM C, BLODAU C, et al. Selective adsorption of dissolved organic matter to mineral soils[J]. Geoderma, 2012, 189: 334-342. |
| [36] | GALINDO C, del NERO M. Molecular level description of the sorptive fractionation of a fulvic acid on aluminum oxide using electrospray ionization Fourier transform mass spectrometry[J]. Environmental Science & Technology, 2014, 48(13): 7 401-7 408. |
| [37] | FLEURY G, del NERO M, BARILLON R. Effect of mineral surface properties (alumina, kaolinite) on the sorptive fractionation mechanisms of soil fulvic acids: molecular-scale ESI-MS studies[J]. Geochimica et Cosmochimica Acta, 2017, 196: 1-17. |
| [38] | WANG X D, XU J, LIU J, et al. Mechanism of Cr(VI) removal by magnetic greigite/biochar composites[J]. Science of the Total Environment, 2020, 700. DOI: 10.1016/j.scitotenv.2019.134414 . |
| [39] | YUE L, LIAN F, HAN Y, et al. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: implications for agronomic benefits and potential risk[J]. Science of the Total Environment, 2019, 656: 9-18. |
| [40] | CHOPPALA G K, BOLAN N S, MEGHARAJ M, et al. The influence of biochar and black carbon on reduction and bioavailability of chromate in soils[J]. Journal of Environmental Quality, 2012, 41(4): 1 175-1 184. |
| [41] | ZHENGYANG E, LIANG J J, DONG Y Q, et al. Different photoreduction processes of Cr(VI) on cellulose-rich and lignin-rich biochar[J]. Environmental Research, 2023, 236.DOI: 10.1016/j.envres.2023.116819 . |
| [42] | WARD C P, CORY R M. Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils[J]. Environmental Science & Technology, 2016, 50(7): 3 545-3 553. |
| [43] | LI L L, WANG X J, FU H Y, et al. Dissolved black carbon facilitates photoreduction of Hg(II) to Hg(0) and reduces mercury uptake by lettuce (Lactuca sativa L.)[J]. Environmental Science & Technology, 2020, 54(18): 11 137-11 145. |
| [44] | LIU Qiang, YUAN Yanfei, LIU Yifan, et al. Research progress: the application of biochar in the remediation of salt-affected soils[J]. Advances in Earth Science, 2022, 37(10): 1 005-1 024. |
| 刘强, 袁延飞, 刘一帆, 等. 生物炭对盐渍化土壤改良的研究进展[J]. 地球科学进展, 2022, 37(10): 1 005-1 024. | |
| [45] | SMITH C R, SLEIGHTER R L, HATCHER P G, et al. Molecular characterization of inhibiting biochar water-extractable substances using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Environmental Science & Technology, 2013, 47(23): 13 294-13 302. |
| [46] | TILLER C L, O’MELIA C R. Natural organic matter and colloidal stability: models and measurements[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1993, 73: 89-102. |
| [47] | SLOWEY A J. Rate of formation and dissolution of mercury sulfide nanoparticles: the dual role of natural organic matter[J]. Geochimica et Cosmochimica Acta, 2010, 74(16): 4 693-4 708. |
| [48] | RIEDEL T, IDEN S, GEILICH J, et al. Changes in the molecular composition of organic matter leached from an agricultural topsoil following addition of biomass-derived black carbon (biochar)[J]. Organic Geochemistry, 2014, 69: 52-60. |
| [49] | CAYUELA M L, van ZWIETEN L, SINGH B P, et al. Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis[J]. Agriculture, Ecosystems & Environment, 2014, 191: 5-16. |
| [50] | SMITH C R, HATCHER P G, KUMAR S, et al. Investigation into the sources of biochar water-soluble organic compounds and their potential toxicity on aquatic microorganisms[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(5): 2 550-2 558. |
| [51] | CHEN S S, ROTARU A E, SHRESTHA P M, et al. Promoting interspecies electron transfer with biochar[J]. Scientific Reports, 2014, 4. DOI: 10.1038/srep05019 . |
| [52] | LEE B M, SEO Y S, HUR J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence EEM-PARAFAC[J]. Water Research, 2015, 73: 242-251. |
| [53] | KLIMM A, BRENNER D, LOK B, et al. Photolytic transformation products of decabromodiphenyl ethane (DBDPE)[J]. Environmental Science & Technology, 2019, 53(11): 6 302-6 309. |
| [54] | HAMEED R, CHENG L L, YANG K, et al. Endogenous release of metals with dissolved organic carbon from biochar: effects of pyrolysis temperature, particle size, and solution chemistry[J]. Environmental Pollution, 2019, 255. DOI: 10.1016/j.envpol.2019.113253 . |
| [55] | LIAO S H, PAN B, LI H, et al. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings[J]. Environmental Science & Technology, 2014, 48(15): 8 581-8 587. |
| [56] | LOU Y M, JOSEPH S, LI L Q, et al. Water extract from straw biochar used for plant growth promotion: an initial test[J]. BioResources, 2015, 11(1): 249-266. |
| [57] | BIAN R J, JOSEPH S, SHI W, et al. Biochar DOM for plant promotion but not residual biochar for metal immobilization depended on pyrolysis temperature[J]. Science of the Total Environment, 2019, 662: 571-580. |
| [58] | FILIMONOVA S, KAUFHOLD S, WAGNER F E, et al. The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol[J]. Geochimica et Cosmochimica Acta, 2016, 180: 284-302. |
| [59] | VIGER M, HANCOCK R D, MIGLIETTA F, et al. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar[J]. GCB Bioenergy, 2015, 7(4): 658-672. |
| [60] | ZHANG X, LI J, FAN W Y, et al. Enhanced photodegradation of extracellular antibiotic resistance genes by dissolved organic matter photosensitization[J]. Environmental Science & Technology, 2019, 53(18): 10 732-10 740. |
| [61] | NORWOOD M J, LOUCHOUARN P, KUO L J, et al. Characterization and biodegradation of water-soluble biomarkers and organic carbon extracted from low temperature chars[J]. Organic Geochemistry, 2013, 56: 111-119. |
| [62] | SUN L N, QIAN J G, BLOUGH N V, et al. Insights into the photoproduction sites of hydroxyl radicals by dissolved organic matter in natural waters[J]. Environmental Science & Technology Letters, 2015, 2(12): 352-356. |
| [63] | JAISWAL A K, ALKAN N, ELAD Y, et al. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease[J]. Scientific Reports, 2020, 10(1).DOI: 10.1038/s41598-020-70882-6 . |
| [64] | SI W, XU H C, KONG M, et al. Effects of molecular weight fractions and chemical properties of time-series cyanobacterial extracellular polymeric substances on the aggregation of lake colloidal particles[J]. Science of the Total Environment, 2019, 692: 1 201-1 208. |
| [65] | XING J, XU G R, LI G B. Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: effect of pyrolysis temperature[J]. Chemosphere, 2020, 250. DOI: 10.1016/j.chemosphere.2020.126184 . |
| [66] | HUANG A Q, ZHI D, TANG H M, et al. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage[J]. Science of the Total Environment, 2020, 720. DOI: 10.1016/j.scitotenv.2020.137560 . |
| [67] | OREN A, CHEFETZ B. Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices[J]. Journal of Environmental Quality, 2012, 41(2): 526-533. |
| [68] | SENESI N. Binding mechanisms of pesticides to soil humic substances[J]. Science of the Total Environment, 1992, 123: 63-76. |
| [69] | TANG J F, LI X H, LUO Y, et al. Spectroscopic characterization of dissolved organic matter derived from different biochars and their Polycylic Aromatic Hydrocarbons (PAHs) binding affinity[J]. Chemosphere, 2016, 152: 399-406. |
| [70] | CHEN Q, MA Y J, DONG J H, et al. The chemical structure characteristics of dissolved black carbon and their binding with phenanthrene[J]. Chemosphere, 2022, 291.DOI: 10.1016/j.chemosphere.2021.132747 . |
| [71] | CHIN Y P, AIKEN G R, DANIELSEN K M. Binding of Pyrene to aquatic and commercial humic substances: the role of molecular weight and aromaticity[J]. Environmental Science & Technology, 1997, 31(6): 1 630-1 635. |
| [72] | BEESLEY L, DICKINSON N. Carbon and trace element fluxes in the pore water of an urban soil following greenwaste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris [J]. Soil Biology and Biochemistry, 2011, 43(1): 188-196. |
| [73] | KIM H B, KIM J G, CHOI J H, et al. Photo-induced redox coupling of dissolved organic matter and iron in biochars and soil system: enhanced mobility of arsenic[J]. Science of the Total Environment, 2019, 689: 1 037-1 043. |
| [74] | SUN S C, BAO Z Y, SUN D Z. Study on emission characteristics and reduction strategy of nitrous oxide during wastewater treatment by different processes[J]. Environmental Science and Pollution Research International, 2015, 22(6): 4 222-4 229. |
| [75] | FANG G D, LIU C, WANG Y J, et al. Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation[J]. Applied Catalysis B: Environmental, 2017, 214: 34-45. |
| [76] | ZHAN M J, YANG X, XIAN Q M, et al. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances[J]. Chemosphere, 2006, 63(3): 378-386. |
| [77] | LI L Y, WEI B, CHENG W, et al. Dual role of dissolved black carbon in sensitized ofloxacin photooxidation: mechanism and influential factors[J]. Science of the Total Environment, 2024, 944. DOI: 10.1016/j.scitotenv.2024.173969 . |
/
| 〈 |
|
〉 |