

内陆水体初级生产力评估方法研究进展
收稿日期: 2021-11-09
修回日期: 2022-05-16
网络出版日期: 2023-02-02
基金资助
国家自然科学基金杰出青年科学基金项目“流域碳氮耦合循环及其生态效应”(42225103)
Estimation of Primary Productivity of Inland Water
Received date: 2021-11-09
Revised date: 2022-05-16
Online published: 2023-02-02
Supported by
the National Science Foundation for Distinguished Young Scholars of China “Coupling cycle of carbon and nitrogen in watershed and its ecological effects”(42225103)
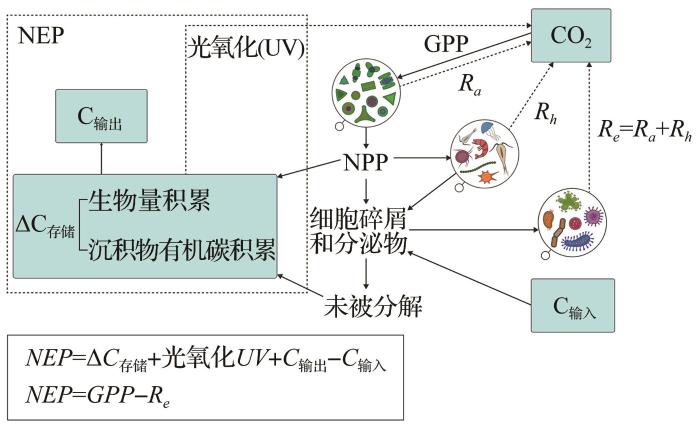
内陆水体是全球碳循环的重要参与者,在调节气候变化方面发挥着关键作用。内陆水体初级生产力指内陆水体(包括湖泊、水库、河流和湿地)中初级生产者(包括浮游藻类植物和挺水、浮水、潜水大型植物)单位时间、单位面积上由光合作用产生的有机物质总量,其大小反映了系统有机碳库和无机碳库之间的定量联系。评估内陆水体初级生产力不仅能帮助解析初级生产者光合作用碳固存机理,也有助于量化内陆水体碳吸收量,进而探知不同区域生态环境差异,揭示内陆水体在全球生态系统碳循环中的重要性。内陆水体初级生产力估算方法较多,包括黑白瓶法、垂向归纳模型法和13C法等,各方法都有其适用范围和局限性,对这些方法的不合理使用制约着对内陆水体初级生产力的变异性及其驱动机制的揭示。通过归纳整理近年来国内外内陆水体初级生产力估算方法,对各个方法的机理、优缺点和适用性进行对比和总结,并着重介绍2种新兴的基于溶解氧浓度或氧同位素的方法(即diel O2技术和18/16O技术),进而为深入开展内陆水体新陈代谢、生产力及养分循环方面研究提供重要技术支撑。
陆瑶 , 黄良波 , 贾珺杰 , 高扬 . 内陆水体初级生产力评估方法研究进展[J]. 地球科学进展, 2023 , 38(1) : 57 -69 . DOI: 10.11867/j.issn.1001-8166.2022.055
Inland water is an important component of the global carbon (C) cycle and plays a key role in regulating climate change. The Primary Productivity (PP) of inland water is defined as the amount of organic matter produced by primary producers in inland water bodies through photosynthesis per unit time and unit area, which reflects the quantitative relationship between the organic and inorganic C pools. The assessment of inland water PP can help analyze the C cycle mechanism of photosynthesis and quantify the C absorption of aquatic ecosystems to examine the differences in the ecological environment in different regions and evaluate the importance of inland water bodies in the global ecosystem C cycle. There are many methods for estimating PP in inland water, including the light-dark bottle incubation method, the vertically generalized production model method, and the 13C method. Each of these have application scopes and limitations. The unreasonable use of PP restricts the understanding of its variability and driving mechanism in inland water bodies. The mechanism, advantages, disadvantages, and applicability of each method are compared by summarizing domestic and international research on PP estimation methods in recent years. Two new methods based on dissolved oxygen concentration or oxygen isotopes, namely, diel O2 technology and 18/16O technology, are introduced. This study serves as a reference for research on inland water metabolism, productivity, and nutrient cycles.

Key words: Primary productivity; Respiration; Carbon cycle; Inland water; Estimation.
| 1 | GAO Yang, JIA Junjie, LU Yao, et al. Determining dominating control mechanisms of inland water carbon cycling processes and associated gross primary productivity on regional and global scales [J]. Earth-Science Reviews, 2020, 213. DOI:10.1016/j.earscirev.2020.103497 . |
| 2 | FALKOWSKI P G, BARBER R T, SMETACEK V. Biogeochemical controls and feedbacks on ocean primary production[J]. Science, 1998, 281(5 374): 200-206. |
| 3 | STAEHR P A, TESTA J M, KEMP W M, et al. The metabolism of aquatic ecosystems: history, applications, and future challenges[J]. Aquatic Sciences, 2012, 74(1): 15-29. |
| 4 | HERNáNDEZ-LEóN S, KOPPELMANN R, FRAILE-NUEZ E, et al. Large deep-sea zooplankton biomass mirrors primary production in the global ocean[J]. Nature Communications, 2020, 11(1). DOI:10.1038/s41467-020-19875-710.1.038/s41467-020-19875-7 . |
| 5 | ENGEL F, ATTERMEYER K, AYALA A I, et al. Phytoplankton gross primary production increases along cascading impoundments in a temperate, low-discharge river: insights from high frequency water quality monitoring[J]. Scientific Reports, 2019, 9(1). DOI:10.1038/s41598-019-43008-w . |
| 6 | CHEN Liwen, ZHANG Guangxin, XU J Y, et al. Human activities and climate variability affecting inland water surface area in a high latitude river basin [J]. Water, 2020, 12(2). DOI:DOI:10.3390/w12020382 . |
| 7 | RAYMOND P A, HARTMANN J, LAUERWALD R, et al. Global carbon dioxide emissions from inland waters[J]. Nature, 2013, 503(7 476): 355-359. |
| 8 | SUTTLE C A. Viruses in the sea [J]. Nature, 2005, 437(7 057): 356-361. |
| 9 | KAUER T, KUTSER T, ARST H, et al. Modelling primary production in shallow well mixed lakes based on MERIS satellite data[J]. Remote Sensing of Environment, 2015, 163: 253-261. |
| 10 | CHEN Shuang, YIN Gaofang, ZHAO Nanjing, et al. Measurement of primary productivity of phytoplankton based on photosynthetic electron transport rate [J]. Acta Optica Sinica, 2018, 38(11): 334-341. |
| 10 | 陈双, 殷高方, 赵南京, 等. 基于光合电子传递速率的浮游植物初级生产力测量[J]. 光学学报, 2018, 38(11): 334-341. |
| 11 | BOGARD M J, VACHON D, ST-GELAIS N F, et al. Using oxygen stable isotopes to quantify ecosystem metabolism in northern lakes[J]. Biogeochemistry, 2017, 133(3): 347-364. |
| 12 | TOBIAS C R, B?HLKE J K, HARVEY J W. The oxygen-18 isotope approach for measuring aquatic metabolism in high productivity waters[J]. Limnology and Oceanography, 2007, 52(4): 1 439-1 453. |
| 13 | HOTCHKISS E R, HALL R O. High rates of daytime respiration in three streams: use of δ18O-O2 and O2 to model diel ecosystem metabolism [J]. Limnology and Oceanography, 2014, 59(3): 798-810. |
| 14 | HOLTGRIEVE G W, SCHINDLER D E, BRANCH T A, et al. Simultaneous quantification of aquatic ecosystem metabolism and reaeration using a Bayesian statistical model of oxygen dynamics[J]. Limnology and Oceanography, 2010, 55(3): 1 047-1 063. |
| 15 | DENG Yubing, ZHANG Yunlin, LI Deping, et al. Temporal and spatial dynamics of phytoplankton primary production in Lake Taihu derived from MODIS data [J]. Remote Sensing, 2017, 9(3). DOI:10.3390/rs9030195 . |
| 16 | YAN Xizhu. The different methods for determing primary production [J]. Chinese Journal of Fisheries, 2000, 13(1): 81-86. |
| 16 | 阎希柱. 初级生产力的不同测定方法[J]. 水产学杂志, 2000, 13(1):81-86. |
| 17 | LOKEN L C, van NIEUWENHUYSE E E, DAHLGREN R A, et al. Assessment of multiple ecosystem metabolism methods in an estuary[J]. Limnology and Oceanography: Methods, 2021, 19(11): 741-757. |
| 18 | PRAVEEN Joshi HS, RAMACHANDRA Naik AT, NARSHIVUDU Daggula, et al. Primary productivity and phytoplankton diversity in Pilikula Lake, Dakshina Kannada dist, Karnataka, India [J]. Journal of Entomology and Zoology Studies, 2019, 7(2): 133-139. |
| 19 | MOLINARI B, STEWART-KOSTER B, ADAME M F, et al. Relationships between algal primary productivity and environmental variables in tropical floodplain wetlands[J]. Inland Waters, 2021, 11(2): 180-190. |
| 20 | TAO Hongbo. Comparison of two estimation methods of vertical primary productivity in Baihua Lake[J]. Modern Agricultural Science and Technology, 2015(19): 224-225, 228. |
| 20 | 陶红波. 百花湖初级生产力的2种估算方法比较[J]. 现代农业科技, 2015(19): 224-225, 228. |
| 21 | ZENG Taiheng, LIU Guoxiang, HU Zhengyu. Estimation of phytoplankton primary production of lakes in the middle and lower reaches of the Yangtze River[J]. Resources and Environment in the Yangtze Basin, 2011, 20(6): 717-722. |
| 21 | 曾台衡, 刘国祥, 胡征宇. 长江中下游湖区浮游植物初级生产力估算[J]. 长江流域资源与环境, 2011, 20(6): 717-722. |
| 22 | ZHANG Yunlin, FENG Sheng, MA Ronghua, et al. Spatial pattern of euphotic depth and estimation of phytoplankton primary production in Lake Taihu in autumn 2004[J]. Journal of Lake Sciences, 2008, 20(3): 380-388. |
| 22 | 张运林, 冯胜, 马荣华, 等. 太湖秋季真光层深度空间分布及浮游植物初级生产力的估算[J]. 湖泊科学, 2008, 20(3): 380-388. |
| 23 | MA Mingzhen. Spatial-temporal pattern of chlorophyll a concentration and its response to changes of nitrogen and phosphorus in Poyang Lake (China) in recent 30 years [D]. Beijing:Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, 2020. |
| 23 | 马明真. 近30年鄱阳湖叶绿素a时空格局变化及其对氮磷变化的响应特征[D]. 北京:中国科学院地理科学与资源研究所, 2020. |
| 24 | YIN Yan, ZHANG Yunlin, SHI Zhiqiang, et al. Estimation of spatial and seasonal changes in phytoplankton primary production in Meiliang Bay, Lake Taihu, based on the Vertically Generalized Production Model and MODIS data[J]. Acta Ecologica Sinica, 2012, 32(11): 3 528-3 537. |
| 24 | 殷燕, 张运林, 时志强, 等. 基于VGPM模型和MODIS数据估算梅梁湾浮游植物初级生产力[J]. 生态学报, 2012, 32(11): 3 528-3 537. |
| 25 | LI Yunliang, ZHANG Yunlin, LIU Mingliang. Calculation and retrieval of euphotic depth of Lake Taihu by remote sensing[J]. Journal of Lake Sciences, 2009, 21(2): 165-172. |
| 25 | 李云亮, 张运林, 刘明亮. 太湖真光层深度的计算及遥感反演[J]. 湖泊科学, 2009, 21(2): 165-172. |
| 26 | CUI Lijuan, QIU Yue, FEI Teng, et al. Using remotely sensed suspended sediment concentration variation to improve management of Poyang Lake, China [J]. Lake and Reservoir Management, 2013, 29(1): 47-60. |
| 27 | CHEN Yuwei, CHEN Kaining, HU Yaohui. Discussion on possible error for phytoplankton chlorophyll-a concentration analysis using hot-ethanol extraction method[J]. Journal of Lake Sciences, 2006, 18(5): 550-552. |
| 27 | 陈宇炜, 陈开宁, 胡耀辉. 浮游植物叶绿素a测定的“热乙醇法”及其测定误差的探讨[J]. 湖泊科学, 2006, 18(5): 550-552. |
| 28 | JIA Junjie, GAO Yang, LU Yao, et al. Trace metal effects on gross primary productivity and its associative environmental risk assessment in a subtropical lake, China [J]. Environmental Pollution, 2020, 259. DOI:10.1016/j.envpol.2019.113848 . |
| 29 | JIA Junjie, GAO Yang, SONG Xianwei, et al. Characteristics of phytoplankton community and water net primary productivity response to the nutrient status of the Poyang Lake and Gan River, China [J]. Ecohydrology, 2019, 12(7). DOI:10.1002/eco.2136 . |
| 30 | WANG Shuoyue, GAO Yang, JIA Junjie, et al. Water level as the key controlling regulator associated with nutrient and gross primary productivity changes in a large floodplain-lake system (Lake Poyang), China [J]. Journal of Hydrology, 2021, 599. DOI:10.1016/j.jhydrol.2021.126414 . |
| 31 | JIA Junjie, WANG Yafeng, LU Yao, et al. Driving mechanisms of gross primary productivity geographical patterns for Qinghai-Tibet Plateau lake systems [J]. The Science of the Total Environment, 2021, 791. DOI:10.1016/j.scitotenv.2021.148286 . |
| 32 | NIELSEN E S. Measurement of the production of organic matter in the sea by means of carbon-14[J]. Nature, 1951, 167(4 252): 684-685. |
| 33 | NIELSEN E S. The use of radio-active carbon (C14) for measuring organic production in the sea[J]. ICES Journal of Marine Science, 1952, 18(2): 117-140. |
| 34 | ICHIMURA S E, SAIJO Y, ARUGA Y. Photosynthetic characteristics of marine phytoplankton and their ecological meaning in the chlorophyll method[J]. Shokubutsugaku Zasshi, 1962, 75(888): 212-220. |
| 35 | LóPEZ-SANDOVAL D C, DELGADO-HUERTAS A, AGUSTí S. The 13C method as a robust alternative to 14C-based measurements of primary productivity in the Mediterranean Sea[J]. Journal of Plankton Research, 2018, 40(5): 544-554. |
| 36 | SUN Ruyong. General ecology [M]. Beijing: Peking University Press, 1993. |
| 36 | 孙儒泳. 普通生态学[M]. 北京:北京大学出版社, 1993. |
| 37 | DOKULIL M T, QIAN K M. Photosynthesis, carbon acquisition and primary productivity of phytoplankton: a review dedicated to Colin Reynolds[J]. Hydrobiologia, 2021, 848(1): 77-94. |
| 38 | HAMA T, MIYAZAKI T, OGAWA Y, et al. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope[J]. Marine Biology, 1983, 73(1): 31-36. |
| 39 | GLASSTONE S, LAIDLER K J, EYRING H. The theory of rate process: the kinetics of chemical reactions, viscosity, diffusion and electrochemical phenomena [R]. McGraw-Hill Book Company, 1941. |
| 40 | MOUSSEAU L, DAUCHEZ S, LEGENDRE L, et al. Photosynthetic carbon uptake by marine phytoplankton: comparison of the stable (13C) and radioactive (14C) isotope methods[J]. Journal of Plankton Research, 1995, 17(7): 1 449-1 460. |
| 41 | SLAWYK G, MINAS M, COLLOS Y, et al. Comparison of radioactive and stable isotope tracer techniques for measuring photosynthesis: 13C and 14C uptake by marine phytoplankton[J]. Journal of Plankton Research, 1984, 6(2): 249-257. |
| 42 | LóPEZ-SANDOVAL D C, DELGADO-HUERTAS A, CARRILLO-de-ALBORNOZ P, et al. Use of cavity ring-down spectrometry to quantify 13C-primary productivity in oligotrophic waters[J]. Limnology and Oceanography: Methods, 2019, 17(2): 137-144. |
| 43 | KISHIMOTO N, YAMAMOTO C, SUZUKI K, et al. Does a decrease in chlorophyll a concentration in lake Biwa mean a decrease in primary productivity by phytoplankton? [J]. Journal of Water and Environment Technology, 2015, 13(1): 1-14. |
| 44 | MIN J, HA S Y, HUR J, et al. Primary productivity and photosynthetic pigment production rates of periphyton and phytoplankton in lake paldang using 13C tracer[J]. Korean Journal of Ecology and Environment, 2019, 52(3): 202-209. |
| 45 | STAEHR P A, BADE D, van de BOGERT M C, et al. Lake metabolism and the diel oxygen technique: state of the science[J]. Limnology and Oceanography: Methods, 2010, 8(11): 628-644. |
| 46 | SARGENT M C, AUSTIN T S. Organic productivity of an atoll[J]. Eos, Transactions American Geophysical Union, 1949, 30(2): 245-249. |
| 47 | ODUM H T, ODUM E P. Trophic structure and productivity of a windward coral reef community on Eniwetok atoll[J]. Ecological Monographs, 1955, 25(3): 291-320. |
| 48 | ODUM H T. Primary production in flowing waters1[J]. Limnology and Oceanography, 1956, 1(2): 102-117. |
| 49 | ODUM H T. Trophic structure and productivity of silver springs, Florida[J]. Ecological Monographs, 1957, 27(1): 55-112. |
| 50 | LOTTIG N R, PHILLIPS J S, BATT R D, et al. Estimating pelagic primary production in lakes: comparison of 14C incubation and free-water O2 approaches[J]. Limnology and Oceanography: Methods, 2022, 20(1): 34-45. |
| 51 | FERNáNDEZ C B, CHMIEL H E, MINAUDO C, et al. Primary and net ecosystem production in a large lake diagnosed from high-resolution oxygen measurements[J]. Water Resources Research, 2021, 57(5). DOI:10.1029/2020WR029283 . |
| 52 | ALFONSO M B, VITALE A J, MENéNDEZ M C, et al. Estimation of ecosystem metabolism from diel oxygen technique in a saline shallow lake: La Salada (Argentina)[J]. Hydrobiologia, 2015, 752(1): 223-237. |
| 53 | WINSLOW L A, ZWART J A, BATT R D, et al. Lake Metabolizer: an R package for estimating lake metabolism from free-water oxygen using diverse statistical models[J]. Inland Waters, 2016, 6(4): 622-636. |
| 54 | BOGARD M J, KUHN C D, JOHNSTON S E, et al. Negligible cycling of terrestrial carbon in many lakes of the arid circumpolar landscape[J]. Nature Geoscience, 2019, 12(3): 180-185. |
| 55 | BOCANIOV S A, SCHIFF S L, SMITH R E H. Plankton metabolism and physical forcing in a productive embayment of a large oligotrophic lake: insights from stable oxygen isotopes[J]. Freshwater Biology, 2012, 57(3): 481-496. |
| 56 | VOGT R J, ST-GELAIS N F, BOGARD M J, et al. Surface water CO2 concentration influences phytoplankton production but not community composition across boreal lakes[J]. Ecology Letters, 2017, 20(11): 1 395-1 404. |
| 57 | XIAO S B, LIU L, WANG W, et al. A Fast-Response Automated Gas Equilibrator\u00a0(FaRAGE) for continuous in situ measurement of CH4 and CO2 dissolved in water[J]. Hydrology and Earth System Sciences, 2020, 24: 3 871-3 880. |
| 58 | COLE J J, PRAIRIE Y T, CARACO N F, et al. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget[J]. Ecosystems, 2007, 10(1): 172-185. |
/
| 〈 |
|
〉 |