1 引 言
硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现。流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] 。流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义。
水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] 。另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] 。但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少。
热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] 。Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少。因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导。
2 理论背景及计算方法
水热体系中Na2 SO4 和K2 SO4 的溶解过程可表示为:
N a 2 S O 4 ( s ) ↔ 2 N a + + S O 4 2 - (1)
K 2 S O 4 ( s ) ↔ 2 K + + S O 4 2 - (2)
K N a 2 S O 4 = a N a + 2 ∙ a S O 4 2 - = m N a γ N a 2 ∙ ( m S O 4 2 - γ S O 4 2 - ) (3)
K K 2 S O 4 = a K + 2 ∙ a S O 4 2 - = m K γ K 2 ∙ ( m S O 4 2 - γ S O 4 2 - ) (4)
式中:K N a 2 S O 4 K K 2 S O 4 ai ,mi 和γ i i 表示Na+ ,K+ 和SO4 2 - 2 SO4 和K2 SO4 的溶解度,就需要同时获得矿物溶解的溶解平衡常数(K sp )和相关物种的活度系数(γ
Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用。Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型。但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] 。
2.1 压力对溶解平衡常数和活度系数的影响
l n K s p T , P = l n K s p T , P 0 - ∆ r V 0 R T P - P 0 (5)
式中:K s p T , P K s p T , P 0 P 和T 分别表示体系的压力和温度,P 0 o C时,P 0 o C时,P 0 ∆ r V 0 2 SO4 (或K2 SO4 )而言:
∆ r V 0 = V 0 N a 2 S O 4 , a q - V 0 ( N a 2 S O 4 , s ) (6)
式中:V 0 N a 2 S O 4 , a q + 和SO4 2 - V 0 ( N a 2 S O 4 , s ) 2 SO4 (或K2 SO4 )的标准偏摩尔体积。25 o C和0.1 MPa时,Na2 SO4 和K2 SO4 的标准偏摩尔体积分别为53.33和65.5 cm3 /mol [38 ] ,且可以不考虑固体矿物的体积随温度压力的变化[37 ] 。
依据Pitzer模型,高压下溶质的活度系数可依据饱和蒸汽压时的活度系数计算[39 ,40 ] :
l n γ P = l n γ P s a t - A ϕ P - A ϕ P s a t × I 1 + b I + 2 b l n ( 1 + b I ) + 2 m β 0 v + β 1 v ( 2 / α 2 I ) 1 - 1 + α I × e x p - α I P - P s a t + 3 m 2 C v ( P - P s a t ) (7)
式中:γ ( P ) γ ( P s a t ) P 和P sat 分别为目标压力和饱和蒸汽压力;A ϕ m 为溶质的质量摩尔浓度,I 为溶液的离子强度;β 0 v β 1 v C v β 0 β 1 C φ α 1/2 ⋅ 1/2 , b = 1.2 kg1/2 。
2.2 高温高压下溶解平衡常数和活度系数的计算
Pitzer模型表示的是物种的吉布斯自由能,经过适当的微分可以得到焓、热容和体积(密度)等性质。相反也可以利用热容、焓和密度等数据来评价温度、压力对模型参数的影响[41 ] 。电解质溶液的密度可以用来计算压力对电解质的偏摩尔体积和Pitzer模型表示的溶质的活度系数的影响[20 ,40 ] 。
V ∅ = 1 m ( 1000 + m M s ρ s o l - 1000 ρ 0 ) (8)
式 中 : V ∅ 3 /mol);m 是电解质的质量摩尔浓度(mol/kg H2 O);Ms 是电解质的摩尔质量(g/mol);ρ s o l ρ 0 3 );其中纯水的密度依据IAPWS97方程来计算[42 ] 。
结合Pitzer模型,电解质的表观摩尔体积可表示为:
V ∅ = V ∅ 0 + v | z + z - | A v l n 1 + b I / 2 b + 2 v + v - m R T ( B v + v + z + m C v ) (9)
B v = β 0 v + β 1 v ( 2 / α 2 I ) 1 - 1 + α I × e x p - α I (10)
式中:V ∅ 0 B v β 0 v β 1 v C v β 1 v [43 ] 。z+ 和z- 分别是阳离子和阴离子的电价,v+ 和v- 分别是阳离子和阴离子的化学计量数,v = v+ + v- 。A v [44 ] 的工作计算得出。I 是溶液的离子强度,R = 8.314 cm3 ·MPa/(K·mol)。
由于电解质的标准偏摩尔体积V ∅ 0 [45 ,46 ] 。
依据公式(9),参考浓度为m r 时溶质的表观摩尔体积为:
V ∅ , m r = V ∅ 0 + v | z + z - | A v l n 1 + b I m r / 2 b + 2 v + v - m r R T ( β 0 v + v + z + m r C v ) (11)
将公式(9)减去公式(11),就可以用参考浓度为m r 时的表观摩尔体积表示任意浓度下溶液的表观摩尔体积:
V ∅ , m = V ∅ , m r + v | z + z - | A v l n 1 + b I m / 2 b - l n 1 + b I m r / 2 b + 2 v + v - R T β 0 v m - m r + z + v + C v m 2 - m r 2 (12)
1000 + m M s m ρ s o l - 1000 m ρ 0
= V m r m r - 1000 m r ρ 0 + v | z + z - | A v l n 1 + b I m / 2 b
- l n 1 + b I m r / 2 b + 2 v + v - R T β 0 v m - m r
+ z + v + C v m 2 - m r 2 (13)
式中:参考浓度m r V ( m r ) β 0 v C v ρ s o l ρ 0 A v β 0 v
V ∅ 0 = V m r m r - 1000 m r ρ 0 - v | z + z - | A v l n 1 + b I m r / 2 b - 2 v + v - m r R T ( β 0 v + v + z + m r C v ) (14)
通过以上过程,利用水热体系中电解质溶液的密度数据,可以拟合获得V ( m r ) β 0 v C v
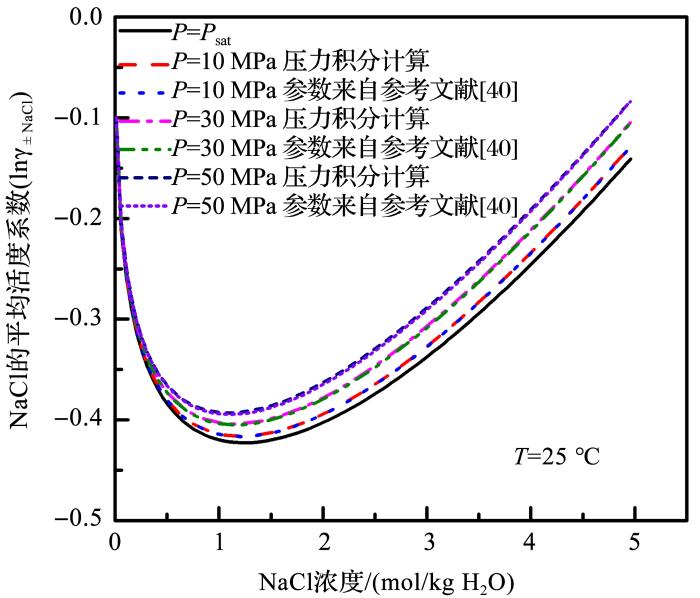
Pitzer等[40 ] 利用水热体系中NaCl溶液的热容和焓等热力学数据确定了0~300 o C,0.1~100.0 MPa,0~6 m范围内NaCl活度系数。Møller[47 ] 确定了0~250 o C,饱和蒸气压下NaCl的活度系数参数;Mao等[25 ] 利用Pitzer模型确定了水热体系中NaCl标准偏摩尔体积和压力对Pitzer参数影响的参数,将二者结合利用公式(7),我们计算了水热体系中NaCl的平均活度系数(图1 )。从图1 可看出,结合饱和蒸汽压下参数与压力积分项计算出来的不同浓度下NaCl的平均活度系数和Pitzer等[40 ] 完整模型参数计算出来的结果吻合得很好,证明了该方法和计算过程的可行性。从图1 还可看出,在NaCl溶液体系中,压力对活度系数有较大的影响,活度系数随着压力的升高而增大。
图1
图1
25 o C 不同压力下,模型计算的NaCl 的平均活度系数随浓度的变化
Fig. 1
The model calculated NaCl mean activity coefficient at 25 o C as a function of concentration under different pressure
3 高温高压条件下Na2 SO4 和K2 SO4 溶解度的计算
对于水热条件下Na2 SO4 体系的密度实验数据文献已有全面而详实的报道,超过1 000个实验数据跨越温度0~300.0 o C,压力0.1~80.0 MPa(表1 )。关于K2 SO4 体系的密度数据相对较少(表2 )。Obšil等[48 ] 和Azizov[58 ] 实验研究了20.0~300.0 o C,10.0~30.0 MPa条件下K2 SO4 溶液的密度;Ellis[53 ] 和Saluja等[54 ] 研究获得了0~200.0 o C,0.1~2.0 MPa条件下K2 SO4 溶液的密度。不同来源的K2 SO4 体系密度数据之间具有较好的一致性,其密度数据的跨度范围为0~300.0 o C,0.1~40.0 MPa。
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为:
V ∅ = V ∅ 0 + 6 A v l n 1 + b I / 2 b + 4 m R T ( β 0 v + 2 m C v ) (15)
V ∅ 0 = V ( m r ) m r - 1000 m r ρ 0 - 6 A v l n 1 + b I m r / 2 b - 4 R T ( m r β 0 v + 2 C v m r 2 ) (16)
其中,参考浓度下的摩尔体积和Pitzer模型压力参数计算如下:
V ( m r ) = a 1 + a 2 T + a 3 T 2 + a 4 T 3 + ( a 5 + a 6 T + a 7 T 2 ) P (17)
β 0 v = a 8 + a 9 T - 227 + a 10 T (18)
C v = a 11 + a 12 T - 227 + a 13 T (19)
式中:T 为开尔文温度;P 表示体系的压力,单位为MPa;a i 2 SO4 和K2 SO4 体系,利用表1 和表2 所列溶液的密度数据,采用非线性最小二乘法,同时拟合获得公式(17)~(19)的参数(表3 )。拟合结果显示,在全部温度压力范围内,模型计算的溶液密度和实验结果的平均偏差分别是0.057% 2 SO4 )和0.047% 2 SO4 ),绝大部分实验数据和模型计算的偏差都在0.1% 2 )。
图2
图2
水热体系中模型计算的Na2 SO4 溶液密度(a )和K2 SO4 溶液密度(b )与实验数据的对比
Fig.2
Plot of density deviations between model calculation and literature data of Na2 SO4 + H2 O (a )and K2 SO4 + H2 O (b ) system
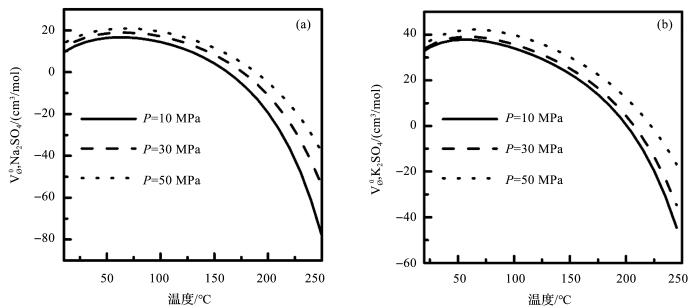
依据公式(16)和表3 中的参数,我们计算不同温度压力条件下Na2 SO4 和K2 SO4 的标准偏摩尔体积。计算结果显示,25 ℃ 2 SO4 和K2 SO4 的标准偏摩尔体积分别为12.26和33.42 cm3 /mol,与实验测得的Na2 SO4 (12.09 cm3 /mol)和K2 SO4 (33.70 cm3 /mol)[59 ] 的结果吻合得很好。与此同时,我们还计算了Na2 SO4 和K2 SO4 的标准偏摩尔体积随温度压力的变化(图3 )。从图3 可看出,Na2 SO4 和K2 SO4 的标准偏摩尔体积随温度强烈的变化,在整个温度范围内,随温度的升高而降低,随压力的增大而增大。
图3
图3
Na2 SO4 (a )和K2 SO4 (b )标准偏摩尔体积随温度的变化
Fig.3
The standard partial molar volumes of Na2 SO4 (a ) and K2 SO4 (b )against temperature and pressure
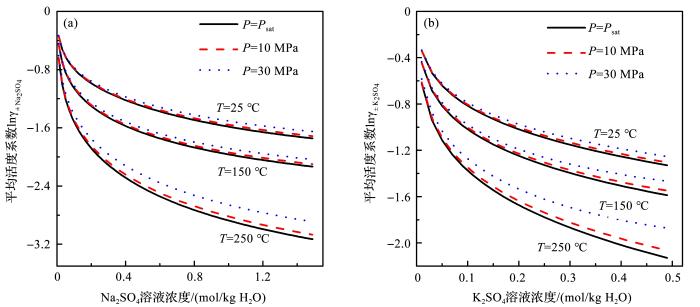
Greenberg等[26 ] 确定了温度0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 活度系数随温度变化的参数。依据公式(7)和以上获得的参数,我们可以评价压力对Na2 SO4 和K2 SO4 活度系数的影响(图4 )。从图4 中可以看出,压力的增大会导致Na2 SO4 和K2 SO4 的平均活度系数的增大,并且随着溶液浓度的增大和温度的增高,这种增加的趋势更明显。对于Na2 SO4 体系,250 o C和30 MPa,1.5 m时Na2 SO4 的平均活度系数比相同温度浓度下,饱和蒸汽压时增加了0.02(图4 a);K2 SO4 体系中,250 o C和30 MPa, 0.5 m时的平均活度系数比饱和蒸汽压时增加了0.03(图4 b)。
图4
图4
不同温度压力下模型计算的Na2 SO4 (a )和K2 SO4 (b )平均活度系数随浓度的变化
Fig. 4
The model calculated Na2 SO4 (a ) and K2 SO4 (b ) mean activity coefficient as a function of concentration at different temperature and pressure
获得了压力对Na2 SO4 和K2 SO4 的标准摩尔体积和活度系数的影响之后,依据公式(4)和公式(5)计算了水热体系中Na2 SO4 和K2 SO4 的溶度积和溶解度。从表4 可以看出,模型计算的结果和实验测得的溶解度之间吻合较好。对于Na2 SO4 体系,Voisin等[60 ] 研究了压力为25 MPa时,水热体系中Na2 SO4 溶解度随温度的变化,并给出了计算Na2 SO4 溶解度的经验公式。从表4 我们发现,本文模型计算的Na2 SO4 溶解度与Voisin等[60 ] 经验公式计算的结果吻合较好。但是对于K2 SO4 体系,高压的实验数据基本都是在超临界条件以上,只能和饱和蒸汽压的实验室数据对比。
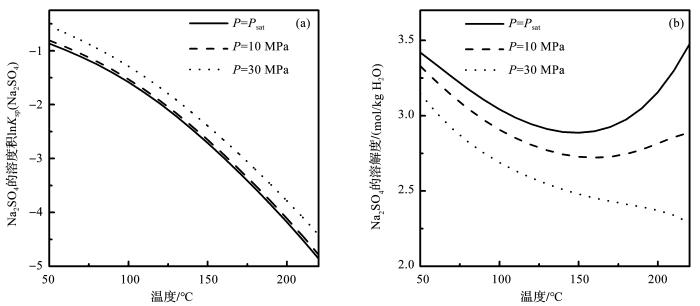
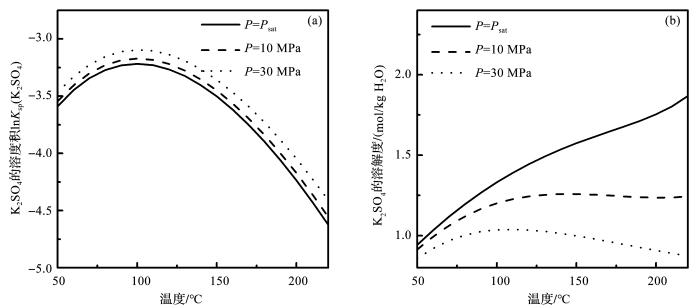
图5 和图6 分别表示水热体系模型计算的Na2 SO4 和K2 SO4 的溶度积的自然对数和溶解度。从图5 a中可以看出,压力能够促进电解质溶解平衡常数的增加,在150 o C,压力为饱和蒸气压,10 MPa和30 MPa时,Na2 SO4 溶解平衡常数的自然对数分别为-2.71,-2.65和-2.55;相同条件下K2 SO4 溶度积的自然对数分别为-3.50,-3.45和-3.36(图6 a)。但是压力对电解质溶解度的影响,却恰好与之相反。从图5 b中可以看出,随着压力的增大,电解质的溶解度却迅速降低。150 o C时,随着压力的增加,Na2 SO4 的溶解度从饱和蒸气压的2.89 mol/kg降低到10 MPa的2.73 mol/kg,最后降低到30 MPa的2.48 mol/kg。在相同温压条件下,K2 SO4 的溶解度分别为1.57,1.26和1.00 mol/kg。随着温度的升高,溶解度的减少量更大(图6 b)。这是因为压力对Na2 SO4 和K2 SO4 的溶度积和平均活度系数都有正向的促进作用,但是压力对Na2 SO4 和K2 SO4 平均活度系数的影响更明显,结果就导致了Na2 SO4 和K2 SO4 溶解度的降低。
图5
图5
Na2 SO4 溶解平衡常数的自然对数(a )和溶解度(b )随温度压力的变化
Fig.5
The natural logarithm of Na2 SO4 solubility product (a ) and solubility (b ) at different temperature and pressure
图6
图6
K2 SO4 溶解平衡常数的自然对数(a )和溶解度(b )随温度压力的变化
Fig.6
The natural logarithm of K2 SO4 solubility product (a ) and solubility (b ) at different temperature and pressure
4 结 论
本文使用Pitzer模型,利用水热体系中Na2 SO4 和K2 SO4 溶液的密度数据,使用非线性最小二乘法拟合,获得了Pitzer模型表示的Na2 SO4 和K2 SO4 的活度系数以及Na2 SO4(aq) 和K2 SO4(aq) 的标准偏摩尔体积随压力变化的模型参数,评价了压力对Na2 SO4 和K2 SO4 的溶度积和活度系数的影响,结果显示压力对Na2 SO4 和K2 SO4 的平均活度系数和溶度积都有正向的促进作用,但是压力对活度系数的影响更大。在此基础上构建了温度到250 o C,压力到40 MPa的Na2 SO4 和K2 SO4 溶解度计算模型。模型计算的Na2 SO4 和K2 SO4 溶解度与已发表文献中的实验数据吻合较好。计算结果显示,Na2 SO4 和K2 SO4 的溶解度随压力的增大而降低,并且随着温度的升高降低的程度也变得更大。
参考文献
View Option
[1]
Xiao Rongge , Zhang Zongheng , Chen Huiquan , et al . Types of geological fluids and ore-forming fluid
[J]. Earth Science Frontiers , 2001 , 8 (4 ): 245 -251 .
[本文引用: 1]
肖荣阁 , 张宗恒 , 陈卉泉 , 等 . 地质流体自然类型与成矿流体类型
[J]. 地学前缘 , 2001 , 8 (4 ): 245 -251 .
[本文引用: 1]
[2]
Xie Yuling , Tian Shihong , Hou Zengqian , et al . Discussion of migration and precipitation mechanics in Muluo REE deposit Miannong country, west Sichuan Province: Evidence from fluid inclusion in bastnaeite
[J]. Acta Petrologica Sinica , 2008 , 24 (3 ):555 -561 .
[本文引用: 1]
谢玉玲 , 田世洪 , 侯增谦 , 等 . 四川冕宁木落稀土矿床稀土元素迁移与沉淀机制: 来自稀土矿物中流体包裹体的证据
[J]. 2008 , 24 (3 ):555 -561 .
[本文引用: 1]
[3]
Li Yongqiang . The Coexistence and Symbiosis Relation Research of Jinding Large-Scale Lead Zinc and the Sulfate Ore Deposit
[D]. Xi'an :Changan University , 2006 .
[本文引用: 1]
李永强 . 兰坪金顶超大型铅锌矿床与硫酸盐矿床共存共生关系研究
[D]. 西安 :长安大学 , 2006 .
[本文引用: 1]
[4]
Xu Jinhong , Zhang Zhengwei , Yang Xiaoyong , et al . The low-temperature mineralization of structurally-controlled fluids in Dahegou antimony ore deposit, Henan Province
[J]. Acta Geologica Sinica , 2017 , 91 (12 ): 2 739 -2 756 .
[本文引用: 1]
徐进鸿 , 张正伟 , 杨晓勇 , 等 . 河南省大河沟锑矿床构造—流体与低温成矿
[J]. 地质学报 , 2017 , 91 (12 ): 2 739 -2 756 .
[本文引用: 1]
[5]
Lu Huanzhang , Fan Hongrui , Ni Pei , et al . Fluind inclusion
[M]. Beijing :Science Press , 2004 .
[本文引用: 1]
卢焕章 , 范宏瑞 , 倪培 , 等 .流体包裹体
[M]. 北京 :科学出版社 , 2004 .
[本文引用: 1]
[6]
Frezzotti M L , Tecce F , Casagli A . Raman spectroscopy for fluid inclusion analysis
[J]. Journal of Geochemical Exploration , 2012 , 112 (1 ): 1 -20 .
[本文引用: 1]
[7]
Li Xiaochun , Fan Hongrui , Hu Fangfang , et al . An analysis of the invidual fluid inclusion by LA-ICP-MS and its application to ore deposit
[J]. Mineral Deposit , 2010 , 29 (6 ): 1 017 - 1 028 .
[本文引用: 1]
李晓春 , 范宏瑞 , 胡芳芳 , 等 . 单个流体包裹体LA-ICP-MS成分分析及在矿床学中的应用
[J]. 矿床地质 , 2010 , 29 (6 ): 1 017 -1 028 .
[本文引用: 1]
[8]
Sun He , Xiao Yilin . Fluid inclusion: Latest development, geological application and prospect
[J]. Advances in Earth Science , 2009 , 24 (10 ): 1 105 -1 121 .
[本文引用: 1]
孙贺 , 肖益林 . 流体包裹体研究:进展、地质应用及展望
[J]. 地球科学进展 , 2009 , 24 (10 ): 1 105 -1 121 .
[本文引用: 1]
[9]
Yao Ying , Sun Qiang . Raman quantitative measurements for carbon isotopic composition in CO2 -rich fluid inclusion: A preliminary study
[J]. Advances in Earth Science , 2016 , 31 (10 ): 1 032 -1 040 .
[本文引用: 1]
药瑛 , 孙樯 . 应用于流体包裹体CO2 碳同位素组成的拉曼光谱定量研究探讨
[J]. 地球科学进展 , 2016 , 31 (10 ): 1 032 -1 040 .
[本文引用: 1]
[10]
Wei Qing , Fan Hongrui , Lan Tingguang , et al . Genesis of Sizhuang gold deposit, Jiaodong peninsula: Evidences from fluid inclusion and quartz solubility modeling
[J]. Acta Petrologica Sinica , 2015 , 31 (4 ):1 049 -1 062 .
[本文引用: 1]
卫清 , 范宏瑞 , 蓝廷广 , 等 . 胶东寺庄金矿床成因: 流体包裹体与石英溶解度证据
[J]. 岩石学报 , 2015 , 31 (4 ): 1 049 -1 062 .
[本文引用: 1]
[11]
Chi Guoxiang , Lai Jianqing . Roles of fluid inclusion in study of mineral deposit
[J]. Mineral Deposit , 2009 , 28 (6 ): 850 -855 .
[本文引用: 1]
池国祥 , 赖健清 . 流体包裹体在矿床研究中的作用
[J]. 矿床地质 , 2009 , 28 (6 ): 850 -855 .
[本文引用: 1]
[12]
Xu Wengang , Fan Hongrui . Ore-forming fluids of the oxidized and reduced porphyry deposits
[J]. Earth Science Frontiers , 2011 , 18 (5 ): 103 -120 .
[本文引用: 1]
徐文刚 , 范宏瑞 . 氧化性和还原性斑岩型矿床流体成矿特征分析
[J]. 地学前缘 , 2011 , 18 (5 ): 103 -120 .
[本文引用: 1]
[13]
Xie Yuling , Hou Zengqian , Yin Shuping , et al . Continuous carbonatitic melt-fluid evolution of a REE mineralization system: Evidence from inclusions in the Maoniuping REE deposit, Western Sichuan, China
[J]. Ore Geology Reviews , 2009 , 36 (1 ): 90 -105 .
[本文引用: 1]
[14]
Linke W F . Solubilities of Inorganic and Metalorganic Compounds (4th ed)
[M]. Washington :American Chemical Society , 1965 .
[本文引用: 1]
[15]
Eysseltová J , Bouaziz R . IUPAC-NIST solubility data series. 93. Potassium sulfate in water
[J]. Journal of Physical and Chemical Reference Data , 2012 , 41 (1 ): 0131031 . DOI: 10.1002/chin.201340220 .
[本文引用: 2]
[16]
Schroeder W C , Gabriel A , Partridge E P . Solubility equilibria of sodium sulfate at temperatures of 150 to 350 °C. I. Effect of sodium hydroxide and sodium chloride
[J]. Journal of the American Chemical Society , 1935 , 57 (9 ): 1 539 -1 546 .
[本文引用: 2]
[17]
Ding X , Zhang T , Zhang S , et al . Experimental determination and modelling of the solubilities of sodium sulfate and potassium sulfate in sub-and supercritical water
[J]. Fluid Phase Equilibria , 2019 , 483 : 31 -51 .
[本文引用: 1]
[18]
Dipippo M M , Sako K , Tester J W . Ternary phase equilibria for the sodium chloride-sodium sulfate-water system at 200 and 250 bar up to 400 °C
[J]. Fluid Phase Equilibria , 1999 , 157 (2 ): 229 -255 .
[本文引用: 1]
[19]
Leusbrock I , Metz S J , Rexwinkel G , et al . Quantitative approaches for the description of solubilities of inorganic compounds in near-critical and supercritical water
[J]. The Journal of Supercritical Fluids , 2008 , 47 (2 ): 117 -127 .
[本文引用: 1]
[20]
Mao S , Peng Q , Wang M , et al . The PVTx properties of aqueous electrolyte solutions containing Li+ , Na+ , K+ , Mg2+ , Ca2+ , Cl− and SO 4 2 - 2 capture and sequestration
[J]. Applied Geochemistry , 2017 , 86 : 105 -120 .
[本文引用: 2]
[21]
Duan Zhenhao . State equation of geological fluid
[J]. Science in China(Series D) , 2010 , 40 (4 ): 393 -413 .
[本文引用: 1]
段振豪 . 地质流体状态方程
[J]. 中国科学: D辑 , 2010 , 40 (4 ): 393 -413 .
[本文引用: 1]
[22]
Zhong R , Brugger J , Tomkins A G , et al . Fate of gold and base metals during metamorphic devolatilization of a pelite
[J]. Geochimica et Cosmochimica Acta , 2015 , 171 : 338 -352 .
[本文引用: 1]
[23]
Pan Aoran , Shan Huimei , Peng Sanxi , et al . Thermodynamic modeling of thioarsenic species distribution in high As groundwater in Hetao Plain
[J]. Advances in Earth Science , 2018 , 33 (11 ): 1 169 -1 180 .
[本文引用: 1]
潘敖然 , 单慧媚 , 彭三曦 , 等 . 基于热力学模拟河套平原高砷地下水中硫代砷形态分布特征
[J]. 地球科学进展 , 2018 , 33 (11 ): 1 169 -1 180 .
[本文引用: 1]
[24]
Hu Q C , Guo H R , Lu X B , et al . Determination of P-V-T-x properties of the CO2 -H2 O system up to 573.15 K and 120 MPa-experiments and model
[J]. Chemical Geology , 2016 , 424 : 60 -72 .
[本文引用: 1]
[25]
Mao S , Duan Z . The P, V, T,x properties of binary aqueous chloride solutions up to T = 573 K and 100 MPa
[J]. The Journal of Chemical Thermodynamics , 2008 , 40 (7 ): 1 046 -1 063 .
[本文引用: 2]
[26]
Greenberg J P , Møller N . The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-K-Ca-Cl-SO4 -H2 O system to high concentration from 0 to 250 °C
[J]. Geochimica et Cosmochimica Acta , 1989 , 53 (10 ): 2 503 -2 518 .
[本文引用: 3]
[27]
Pabalan R T , Pitzer K S . Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na-K-Mg-Cl-SO4 -OH-H2 O
[J]. Geochimica et Cosmochimica Acta , 1987 , 51 (9 ): 2 429 -2 443 .
[本文引用: 2]
[28]
Pitzer K S . Thermodynamics of electrolytes. I. Theoretical basis and general equations
[J]. The Journal of Physical Chemistry , 1973 , 77 (2 ): 268 -277 .
[本文引用: 1]
[29]
Wang L , Zhang W , Yang B , et al . Solubility measurements in Na-F-CO3 -HCO3 -H2 O system at (308.15 and 323.15) K and development of a Pitzer-based equilibrium model for the Na-F-Cl-SO4 -CO3 -HCO3 -H2 O system
[J]. The Journal of Chemical Thermodynamics , 2019 , 131 : 88 -96 .
[本文引用: 1]
[30]
Parkhurst D L , Appelo C A J . Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation
Batch-Reaction , Transport One-Dimensional , and Inverse Geochemical Calculations
[R]. U.S. Geological Survey , 2013 .
[本文引用: 1]
[31]
Kulik D A , Wagner T , Dmytrieva S V , et al . GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes
[J]. Computational Geosciences , 2013 , 17 (1 ): 1 -24 .
[本文引用: 1]
[32]
Dai Z , Kan A , Zhang F , et al . A thermodynamic model for the solubility prediction of barite, calcite, gypsum, and anhydrite, and the association constant sstimation of CaSO4 (0) ion pair up to 250 °C and 22000 psi
[J]. Journal of Chemical and Engineering Data , 2015 , 60 (3 ): 766 -774 .
[本文引用: 1]
[33]
Shi W , Kan A , Fan C , et al . Solubility of barite up to 250 °C and 1500 bar in up to 6 m NaCl solution
[J]. Industrial and Engineering Chemistry Research , 2012 , 51 (7 ): 3 119 -3 128 .
[本文引用: 1]
[34]
Dai Z , Kan A , Shi W , et al . Calcite and barite solubility measurements in mixed electrolyte solutions and development of a comprehensive model for water-mineral-gas equilibrium of the Na-K-Mg-Ca-Ba-Sr-Cl-SO4 -CO3 -HCO3 -CO2(aq) -H2 O System up to 250 °C and 1500 bar
[J]. Industrial and Engineering Chemistry Research , 2017 , 56 (23 ): 6 548 -6 561 .
[本文引用: 1]
[35]
Millero F J . The effect of pressure on the solubility of minerals in water and seawater
[J]. Geochimica et Cosmochimica Acta , 1982 , 46 (1 ): 11 -22 .
[本文引用: 1]
[36]
Appelo C A J , Parkhurst D L , Post V E A . Equations for calculating hydrogeochemical reactions of minerals and gases such as CO2 at high pressures and temperatures
[J]. Geochimica et Cosmochimica Acta , 2014 , 125 : 49 -67 .
[本文引用: 2]
[37]
Monnin C . A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200 °C and to 1 kbar
[J]. Chemical Geology , 1999 , 153 (1 /4 ): 187 -209 .
[本文引用: 2]
[38]
Robie R A , Hemmingway B , Fisher J R . Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 bar (10⁵ pascals) Pressure and at Higher Temperature
[R]. Denver,CO : U .S. Geological Survey , 1978 .
[本文引用: 1]
[39]
Rogers P S Z , Pitzer K S . Volumetric properties of aqueous sodium vhloride solutions
[J]. Journal of Physical and Chemical Reference Data , 1982 , 11 (1 ): 15 -81 .
[本文引用: 1]
[40]
Pitzer K S , Peiper J C , Busey R H . Thermodynamic properties of aqueous sodium chloride solutions
[J]. Journal of Physical and Chemical Reference Data , 1984 , 13 (1 ): 1 -102 .
[本文引用: 4]
[41]
Königsberger E , Eriksson G , May P M , et al . Comprehensive model of synthetic Bayer liquors. Part 1. Overview
[J]. Industrial and Engineering Chemistry Research , 2005 , 44 (15 ):5 805 -5 814 .
[本文引用: 1]
[42]
Wagner W , Kruse A , Kurtzschmar H J . Properties of Water and steam: The Industrial Standard IAPWS-IF97 for the Thermodynamic Properties and Supplementary Equations for Other Properties: Tables Based on These Equations
[M]. Berlin :Springer-Verlag Berlin , 1998 .
[本文引用: 1]
[43]
Pabalan R T , Pitzer K S . Thermodynamics of NaOH(aq) in hydrothermal solutions
[J]. Geochimica et Cosmochimica Acta , 1987 , 51 (4 ): 829 -837 .
[本文引用: 1]
[44]
Bradley D J , Pitzer K S . Thermodynamics of electrolytes. 12. Dielectric properties of water and Debye-Hückel parameters to 350 °C and 1 kbar
[J]. Journal of Physical Chemistry , 1979 , 83 (12 ): 1 599 -1 603 .
[本文引用: 1]
[45]
Archer D G . Thermodynamic properties of the KCl+H2 O system
[J]. Journal of Physical and Chemical Reference Data , 1999 , 28 (1 ): 1 -17 .
[本文引用: 1]
[46]
Rowland D , May P M . A Pitzer-based characterization of aqueous magnesium chloride, calcium chloride and potassium iodide solution densities to high temperature and pressure
[J]. Fluid Phase Equilibria , 2013 , 338 : 54 -62 .
[本文引用: 1]
[47]
Møller N . The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-Ca-Cl-SO4 -H2 O system, to high temperature and concentration
[J]. Geochimica et Cosmochimica Acta , 1988 , 52 (4 ): 821 -837 .
[本文引用: 1]
[48]
Obšil M , Majer V , Hefter G T , et al . Densities and apparent molar volumes of Na2 SO4(aq) and K2 SO4(aq) at temperatures from 298 K to 573 K and at pressures up to 30 MPa
[J]. Journal of Chemical and Engineering Data , 1997 , 42 (1 ): 137 -142 .
[本文引用: 3]
[49]
Al Ghafri S Z , Maitland G C , Trusler J P M . Densities of SrCl2(aq) , Na2 SO4(aq) , NaHCO3(aq) , and two synthetic reservoir brines at temperatures between (298 and 473) K, pressures up to 68.5 MPa, and molalities up to 3 mol·kg-1
[J]. Journal of Chemical and Engineering Data , 2013 , 58 (2 ): 402 -412 .
[本文引用: 1]
[50]
Apelblat A , Manzurola E , Orekhova Z . Thermodynamic properties of aqueous electrolyte solutions. volumetric and compressibility studies in 0.1 mol. kg-1 , 0.5 mol·kg-1 , and 1.0 mol·kg-1 sodium carbonate and sodium sulfate solutions at temperatures from 278.15 K to 323.15 K
[J]. Journal of Chemical and Engineering Data , 2009 , 54 (9 ): 2 550 -2 561 .
[本文引用: 1]
[51]
Saluja P P S , Pitzer K S , Phutela R C . High-temperature thermodynamic properties of several 1∶1 electrolytes
[J]. Canadian Journal of Chemistry , 1986 , 64 (7 ): 1 328 -1 335 .
[本文引用: 1]
[52]
Chen C T , Emmet R T , Millero F J . The apparent molal volumes of aqueous solutions of sodium chloride, potassium chloride, magnesium chloride, sodium sulfate, and magnesium sulfate from 0 to 1000 bars at 0, 25, and 50 °C
[J]. Journal of Chemical and Engineering Data , 1977 , 22 (2 ): 201 -207 .
[本文引用: 1]
[53]
Ellis A J . Partial molal volumes in high-temperature water. Part III. halide and oxyanion salts
[J]. Journal of the Chemical Society A: Inorganic, Physical, Theoretical , 1968 . DOI:10.1039/j19680001138 .
[本文引用: 3]
[54]
Saluja P P S , Lemire R J , Leblanc J C . High-temperature thermodynamics of aqueous alkali-metal salts
[J]. The Journal of Chemical Thermodynamics , 1992 , 24 (2 ): 181 -203 .
[本文引用: 3]
[55]
Zezin D , Driesner T , Sanchez-Valle C . Volumetric properties of Na2 SO4 -H2 O and Na2 SO4 -NaCl-H2 O solutions to 523.15 K, 70 MPa
[J]. Journal of Chemical and Engineering Data , 2015 , 60 (4 ): 1 181 -1 192 .
[本文引用: 1]
[56]
Azizov N D , Akhundov T S . The bulk properties of the Na2 SO4 -H2 O system in a wide range of the parameters of state
[J]. High Temperature , 2000 , 38 (2 ): 203 -209 .
[本文引用: 1]
[57]
Laliberté M . A model for calculating the heat capacity of aqueous solutions, with updated density and viscosity Data
[J]. Journal of Chemical and Engineering Data , 2009 , 54 (6 ): 1 725 -1 760 .
[本文引用: 2]
[58]
Azizov N D . The density and partial properties of K2 SO4 -H2 O solutions from room temperature to 573 K
[J]. Zhurnal Neorganicheskoi Khimii , 1998 , 43 (2 ): 323 -327 .
[本文引用: 2]
[59]
Pearce J N , Eckstrom H C . Vapor pressures and partial molal volumes of aqueous solutions of the alkali sulfates at 25°
[J]. Journal of the American Chemical Society , 1937 , 59 (12 ): 2 689 -2 691 .
[本文引用: 1]
[60]
Voisin T , Erriguible A , Philippot G , et al . Investigation of the precipitation of Na2 SO4 in supercritical water
[J]. Chemical Engineering Science , 2017 , 174 : 268 -276 .
[本文引用: 3]
Types of geological fluids and ore-forming fluid
1
2001
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
地质流体自然类型与成矿流体类型
1
2001
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Discussion of migration and precipitation mechanics in Muluo REE deposit Miannong country, west Sichuan Province: Evidence from fluid inclusion in bastnaeite
1
2008
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
四川冕宁木落稀土矿床稀土元素迁移与沉淀机制: 来自稀土矿物中流体包裹体的证据
1
2008
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
The Coexistence and Symbiosis Relation Research of Jinding Large-Scale Lead Zinc and the Sulfate Ore Deposit
1
2006
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
兰坪金顶超大型铅锌矿床与硫酸盐矿床共存共生关系研究
1
2006
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
The low-temperature mineralization of structurally-controlled fluids in Dahegou antimony ore deposit, Henan Province
1
2017
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
河南省大河沟锑矿床构造—流体与低温成矿
1
2017
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Fluind inclusion
1
2004
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
流体包裹体
1
2004
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Raman spectroscopy for fluid inclusion analysis
1
2012
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
An analysis of the invidual fluid inclusion by LA-ICP-MS and its application to ore deposit
1
2010
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
单个流体包裹体LA-ICP-MS成分分析及在矿床学中的应用
1
2010
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Fluid inclusion: Latest development, geological application and prospect
1
2009
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
流体包裹体研究:进展、地质应用及展望
1
2009
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Raman quantitative measurements for carbon isotopic composition in CO2 -rich fluid inclusion: A preliminary study
1
2016
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
应用于流体包裹体CO2 碳同位素组成的拉曼光谱定量研究探讨
1
2016
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Genesis of Sizhuang gold deposit, Jiaodong peninsula: Evidences from fluid inclusion and quartz solubility modeling
1
2015
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
胶东寺庄金矿床成因: 流体包裹体与石英溶解度证据
1
2015
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Roles of fluid inclusion in study of mineral deposit
1
2009
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
流体包裹体在矿床研究中的作用
1
2009
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Ore-forming fluids of the oxidized and reduced porphyry deposits
1
2011
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
氧化性和还原性斑岩型矿床流体成矿特征分析
1
2011
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Continuous carbonatitic melt-fluid evolution of a REE mineralization system: Evidence from inclusions in the Maoniuping REE deposit, Western Sichuan, China
1
2009
... 硫酸盐流体是地质流体的一个重要类型,在海陆相各环境中广泛存在[1 ] ,在四川冕宁木落稀土矿床[2 ] 、兰坪金顶超大型铅锌矿床[3 ] 和河南省大河沟锑矿[4 ] 都有发现.流体包裹体是圈闭在矿物晶格缺陷,或其窝穴中保存下来的古流体,是成岩成矿流体的最直观体现[5 ] .流体包裹体的一项重要研究内容是包裹体盐度的确定[6 ,7 ,8 ,9 ] ,成矿热液中硫酸盐(Na2 SO4 /K2 SO4 )溶解度的研究,对反映成矿流体演化过程[10 ] 、矿床类型的划分[11 ] 、流体的氧化还原状态[12 ] 和成矿元素的迁移富集规律[13 ] 具有重要的指示意义. ...
Solubilities of Inorganic and Metalorganic Compounds (4th ed)
1
1965
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
IUPAC-NIST solubility data series. 93. Potassium sulfate in water
2
2012
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
...
P =
P sat 50.00 0.95 0.94 [15 ] 80.00 1.23 1.20 100.0 1.40 1.33 150.0 1.69 1.57 190.0 1.97 1.71 Na2 SO4 ...
Solubility equilibria of sodium sulfate at temperatures of 150 to 350 °C. I. Effect of sodium hydroxide and sodium chloride
2
1935
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
... P =P sat
60.0 3.15 3.34 [16 ,27 ] 100.0 2.99 3.04 120.0 2.95 2.94 140.7 2.96 2.89 200.7 3.15 3.16 K2 SO4 ...
Experimental determination and modelling of the solubilities of sodium sulfate and potassium sulfate in sub-and supercritical water
1
2019
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
Ternary phase equilibria for the sodium chloride-sodium sulfate-water system at 200 and 250 bar up to 400 °C
1
1999
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
Quantitative approaches for the description of solubilities of inorganic compounds in near-critical and supercritical water
1
2008
... 水热体系中Na2 SO4 /K2 SO4 溶解度的研究已经取得了很多非常重要的成果,但是大部分实验数据的温度范围都是在100 o C以内[14 ] ,温度在100 o C以上的数据也多是在饱和蒸汽压条件下[15 ,16 ] .另外,由于Na2 SO4 /K2 SO4 等无机超临界流体的特殊性质,化学家们研究了超临界条件下(温度≥374 o C、压力≥22 MPa)Na2 SO4 和K2 SO4 的溶解度,并给出了计算其溶解度的相关经验模型[17 ,18 ,19 ] .但是对于温度≤250 o C的低温热液矿床内的硫酸盐流体性质的研究很少. ...
The PVTx properties of aqueous electrolyte solutions containing Li+ , Na+ , K+ , Mg2+ , Ca2+ , Cl? and SO 4 2 - under conditions of CO2 capture and sequestration
2
2017
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
... Pitzer模型表示的是物种的吉布斯自由能,经过适当的微分可以得到焓、热容和体积(密度)等性质.相反也可以利用热容、焓和密度等数据来评价温度、压力对模型参数的影响[41 ] .电解质溶液的密度可以用来计算压力对电解质的偏摩尔体积和Pitzer模型表示的溶质的活度系数的影响[20 ,40 ] . ...
State equation of geological fluid
1
2010
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
地质流体状态方程
1
2010
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
Fate of gold and base metals during metamorphic devolatilization of a pelite
1
2015
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
Thermodynamic modeling of thioarsenic species distribution in high As groundwater in Hetao Plain
1
2018
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
基于热力学模拟河套平原高砷地下水中硫代砷形态分布特征
1
2018
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
Determination of P-V-T-x properties of the CO2 -H2 O system up to 573.15 K and 120 MPa-experiments and model
1
2016
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
The P, V, T,x properties of binary aqueous chloride solutions up to T = 573 K and 100 MPa
2
2008
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
... Pitzer等[40 ] 利用水热体系中NaCl溶液的热容和焓等热力学数据确定了0~300 o C,0.1~100.0 MPa,0~6 m范围内NaCl活度系数.Møller[47 ] 确定了0~250 o C,饱和蒸气压下NaCl的活度系数参数;Mao等[25 ] 利用Pitzer模型确定了水热体系中NaCl标准偏摩尔体积和压力对Pitzer参数影响的参数,将二者结合利用公式(7),我们计算了水热体系中NaCl的平均活度系数(图1 ).从图1 可看出,结合饱和蒸汽压下参数与压力积分项计算出来的不同浓度下NaCl的平均活度系数和Pitzer等[40 ] 完整模型参数计算出来的结果吻合得很好,证明了该方法和计算过程的可行性.从图1 还可看出,在NaCl溶液体系中,压力对活度系数有较大的影响,活度系数随着压力的升高而增大. ...
The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-K-Ca-Cl-SO4 -H2 O system to high concentration from 0 to 250 °C
3
1989
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
... Greenberg等[26 ] 确定了温度0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 活度系数随温度变化的参数.依据公式(7)和以上获得的参数,我们可以评价压力对Na2 SO4 和K2 SO4 活度系数的影响(图4 ).从图4 中可以看出,压力的增大会导致Na2 SO4 和K2 SO4 的平均活度系数的增大,并且随着溶液浓度的增大和温度的增高,这种增加的趋势更明显.对于Na2 SO4 体系,250 o C和30 MPa,1.5 m时Na2 SO4 的平均活度系数比相同温度浓度下,饱和蒸汽压时增加了0.02(图4 a);K2 SO4 体系中,250 o C和30 MPa, 0.5 m时的平均活度系数比饱和蒸汽压时增加了0.03(图4 b). ...
Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na-K-Mg-Cl-SO4 -OH-H2 O
2
1987
... 热力学计算是模拟物质迁移转化、成矿流体演化,及成矿流体热力学性质最常用也是最强有力的工具之一[20 ,21 ,22 ,23 ] ,特别是在实验结果较少的温压范围内起着重要的作用,且在H2 O,NaCl-H2 O以及NaCl-H2 O-CO2 流体热力学性质方面已经取得了丰硕的成果[24 ,25 ] .Greenberg等[26 ] 和Pabalan等[27 ] 总结了文献中Na2 SO4 和K2 SO4 体系的溶解度等热力学数据,利用Pitzer模型建立了0~250 o C,饱和蒸汽压下Na2 SO4 和K2 SO4 溶解度计算模型,但是对于更高压力条件下硫酸盐溶解度的研究还很少.因此,本文拟通过热力学计算研究低温热液水热体系中Na2 SO4 和K2 SO4 的溶解度,力图为低温成矿热液中硫酸盐流体包裹体盐度的研究提供一定的指导. ...
... P =P sat
60.0 3.15 3.34 [16 ,27 ] 100.0 2.99 3.04 120.0 2.95 2.94 140.7 2.96 2.89 200.7 3.15 3.16 K2 SO4 ...
Thermodynamics of electrolytes. I. Theoretical basis and general equations
1
1973
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
Solubility measurements in Na-F-CO3 -HCO3 -H2 O system at (308.15 and 323.15) K and development of a Pitzer-based equilibrium model for the Na-F-Cl-SO4 -CO3 -HCO3 -H2 O system
1
2019
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
Inverse Geochemical Calculations
1
2013
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes
1
2013
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
A thermodynamic model for the solubility prediction of barite, calcite, gypsum, and anhydrite, and the association constant sstimation of CaSO4 (0) ion pair up to 250 °C and 22000 psi
1
2015
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
Solubility of barite up to 250 °C and 1500 bar in up to 6 m NaCl solution
1
2012
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
Calcite and barite solubility measurements in mixed electrolyte solutions and development of a comprehensive model for water-mineral-gas equilibrium of the Na-K-Mg-Ca-Ba-Sr-Cl-SO4 -CO3 -HCO3 -CO2(aq) -H2 O System up to 250 °C and 1500 bar
1
2017
... Pitzer[28 ] 模型被广泛应用于水热体系中物质活度系数的计算[29 ] ,被PHREEQC[30 ] ,GEM-Selektor[31 ] 和ScaleSoftPitzer[32 ] 等世界知名的地球化学计算软件所采用.Greenberg等[26 ] 利用Pitzer模型建立了0~250 o C,饱和蒸气压下Na2 SO4 和K2 SO4 的溶解度计算模型.但是,Shi等[33 ] 发现不考虑压力情况下重晶石的溶解度要比实验结果低27% [34 ] . ...
The effect of pressure on the solubility of minerals in water and seawater
1
1982
... 溶解平衡常数随压力的变化可表示为[35 ,36 ,37 ] : ...
Equations for calculating hydrogeochemical reactions of minerals and gases such as CO2 at high pressures and temperatures
2
2014
... 溶解平衡常数随压力的变化可表示为[35 ,36 ,37 ] : ...
... 电解质的表观摩尔体积可以表示为[36 ] : ...
A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200 °C and to 1 kbar
2
1999
... 溶解平衡常数随压力的变化可表示为[35 ,36 ,37 ] : ...
... 式中:V 0 N a 2 S O 4 , a q + 和SO4 2 - V 0 ( N a 2 S O 4 , s ) 2 SO4 (或K2 SO4 )的标准偏摩尔体积.25 o C和0.1 MPa时,Na2 SO4 和K2 SO4 的标准偏摩尔体积分别为53.33和65.5 cm3 /mol [38 ] ,且可以不考虑固体矿物的体积随温度压力的变化[37 ] . ...
Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 bar (10? pascals) Pressure and at Higher Temperature
1
1978
... 式中:V 0 N a 2 S O 4 , a q + 和SO4 2 - V 0 ( N a 2 S O 4 , s ) 2 SO4 (或K2 SO4 )的标准偏摩尔体积.25 o C和0.1 MPa时,Na2 SO4 和K2 SO4 的标准偏摩尔体积分别为53.33和65.5 cm3 /mol [38 ] ,且可以不考虑固体矿物的体积随温度压力的变化[37 ] . ...
Volumetric properties of aqueous sodium vhloride solutions
1
1982
... 依据Pitzer模型,高压下溶质的活度系数可依据饱和蒸汽压时的活度系数计算[39 ,40 ] : ...
Thermodynamic properties of aqueous sodium chloride solutions
4
1984
... 依据Pitzer模型,高压下溶质的活度系数可依据饱和蒸汽压时的活度系数计算[39 ,40 ] : ...
... Pitzer模型表示的是物种的吉布斯自由能,经过适当的微分可以得到焓、热容和体积(密度)等性质.相反也可以利用热容、焓和密度等数据来评价温度、压力对模型参数的影响[41 ] .电解质溶液的密度可以用来计算压力对电解质的偏摩尔体积和Pitzer模型表示的溶质的活度系数的影响[20 ,40 ] . ...
... Pitzer等[40 ] 利用水热体系中NaCl溶液的热容和焓等热力学数据确定了0~300 o C,0.1~100.0 MPa,0~6 m范围内NaCl活度系数.Møller[47 ] 确定了0~250 o C,饱和蒸气压下NaCl的活度系数参数;Mao等[25 ] 利用Pitzer模型确定了水热体系中NaCl标准偏摩尔体积和压力对Pitzer参数影响的参数,将二者结合利用公式(7),我们计算了水热体系中NaCl的平均活度系数(图1 ).从图1 可看出,结合饱和蒸汽压下参数与压力积分项计算出来的不同浓度下NaCl的平均活度系数和Pitzer等[40 ] 完整模型参数计算出来的结果吻合得很好,证明了该方法和计算过程的可行性.从图1 还可看出,在NaCl溶液体系中,压力对活度系数有较大的影响,活度系数随着压力的升高而增大. ...
... [40 ]完整模型参数计算出来的结果吻合得很好,证明了该方法和计算过程的可行性.从图1 还可看出,在NaCl溶液体系中,压力对活度系数有较大的影响,活度系数随着压力的升高而增大. ...
Comprehensive model of synthetic Bayer liquors. Part 1. Overview
1
2005
... Pitzer模型表示的是物种的吉布斯自由能,经过适当的微分可以得到焓、热容和体积(密度)等性质.相反也可以利用热容、焓和密度等数据来评价温度、压力对模型参数的影响[41 ] .电解质溶液的密度可以用来计算压力对电解质的偏摩尔体积和Pitzer模型表示的溶质的活度系数的影响[20 ,40 ] . ...
Properties of Water and steam: The Industrial Standard IAPWS-IF97 for the Thermodynamic Properties and Supplementary Equations for Other Properties: Tables Based on These Equations
1
1998
... 式 中 : V ∅ 3 /mol);m 是电解质的质量摩尔浓度(mol/kg H2 O);Ms 是电解质的摩尔质量(g/mol);ρ s o l ρ 0 3 );其中纯水的密度依据IAPWS97方程来计算[42 ] . ...
Thermodynamics of NaOH(aq) in hydrothermal solutions
1
1987
... 式中:V ∅ 0 B v β 0 v β 1 v C v β 1 v [43 ] .z+ 和z- 分别是阳离子和阴离子的电价,v+ 和v- 分别是阳离子和阴离子的化学计量数,v = v+ + v- .A v [44 ] 的工作计算得出.I 是溶液的离子强度,R = 8.314 cm3 ·MPa/(K·mol). ...
Thermodynamics of electrolytes. 12. Dielectric properties of water and Debye-Hückel parameters to 350 °C and 1 kbar
1
1979
... 式中:V ∅ 0 B v β 0 v β 1 v C v β 1 v [43 ] .z+ 和z- 分别是阳离子和阴离子的电价,v+ 和v- 分别是阳离子和阴离子的化学计量数,v = v+ + v- .A v [44 ] 的工作计算得出.I 是溶液的离子强度,R = 8.314 cm3 ·MPa/(K·mol). ...
Thermodynamic properties of the KCl+H2 O system
1
1999
... 由于电解质的标准偏摩尔体积V ∅ 0 [45 ,46 ] . ...
A Pitzer-based characterization of aqueous magnesium chloride, calcium chloride and potassium iodide solution densities to high temperature and pressure
1
2013
... 由于电解质的标准偏摩尔体积V ∅ 0 [45 ,46 ] . ...
The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-Ca-Cl-SO4 -H2 O system, to high temperature and concentration
1
1988
... Pitzer等[40 ] 利用水热体系中NaCl溶液的热容和焓等热力学数据确定了0~300 o C,0.1~100.0 MPa,0~6 m范围内NaCl活度系数.Møller[47 ] 确定了0~250 o C,饱和蒸气压下NaCl的活度系数参数;Mao等[25 ] 利用Pitzer模型确定了水热体系中NaCl标准偏摩尔体积和压力对Pitzer参数影响的参数,将二者结合利用公式(7),我们计算了水热体系中NaCl的平均活度系数(图1 ).从图1 可看出,结合饱和蒸汽压下参数与压力积分项计算出来的不同浓度下NaCl的平均活度系数和Pitzer等[40 ] 完整模型参数计算出来的结果吻合得很好,证明了该方法和计算过程的可行性.从图1 还可看出,在NaCl溶液体系中,压力对活度系数有较大的影响,活度系数随着压力的升高而增大. ...
Densities and apparent molar volumes of Na2 SO4(aq) and K2 SO4(aq) at temperatures from 298 K to 573 K and at pressures up to 30 MPa
3
1997
... 对于水热条件下Na2 SO4 体系的密度实验数据文献已有全面而详实的报道,超过1 000个实验数据跨越温度0~300.0 o C,压力0.1~80.0 MPa(表1 ).关于K2 SO4 体系的密度数据相对较少(表2 ).Obšil等[48 ] 和Azizov[58 ] 实验研究了20.0~300.0 o C,10.0~30.0 MPa条件下K2 SO4 溶液的密度;Ellis[53 ] 和Saluja等[54 ] 研究获得了0~200.0 o C,0.1~2.0 MPa条件下K2 SO4 溶液的密度.不同来源的K2 SO4 体系密度数据之间具有较好的一致性,其密度数据的跨度范围为0~300.0 o C,0.1~40.0 MPa. ...
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
... Summary of density data of K
2 SO
4 -H
2 O system
Table 2 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 0~90.0 0.1 [57 ] 20.0~300.0 5.0~40.0 [58 ]
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为: ...
Densities of SrCl2(aq) , Na2 SO4(aq) , NaHCO3(aq) , and two synthetic reservoir brines at temperatures between (298 and 473) K, pressures up to 68.5 MPa, and molalities up to 3 mol·kg-1
1
2013
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
Thermodynamic properties of aqueous electrolyte solutions. volumetric and compressibility studies in 0.1 mol. kg-1 , 0.5 mol·kg-1 , and 1.0 mol·kg-1 sodium carbonate and sodium sulfate solutions at temperatures from 278.15 K to 323.15 K
1
2009
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
High-temperature thermodynamic properties of several 1∶1 electrolytes
1
1986
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
The apparent molal volumes of aqueous solutions of sodium chloride, potassium chloride, magnesium chloride, sodium sulfate, and magnesium sulfate from 0 to 1000 bars at 0, 25, and 50 °C
1
1977
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
Partial molal volumes in high-temperature water. Part III. halide and oxyanion salts
3
1968
... 对于水热条件下Na2 SO4 体系的密度实验数据文献已有全面而详实的报道,超过1 000个实验数据跨越温度0~300.0 o C,压力0.1~80.0 MPa(表1 ).关于K2 SO4 体系的密度数据相对较少(表2 ).Obšil等[48 ] 和Azizov[58 ] 实验研究了20.0~300.0 o C,10.0~30.0 MPa条件下K2 SO4 溶液的密度;Ellis[53 ] 和Saluja等[54 ] 研究获得了0~200.0 o C,0.1~2.0 MPa条件下K2 SO4 溶液的密度.不同来源的K2 SO4 体系密度数据之间具有较好的一致性,其密度数据的跨度范围为0~300.0 o C,0.1~40.0 MPa. ...
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
... Summary of density data of K
2 SO
4 -H
2 O system
Table 2 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 0~90.0 0.1 [57 ] 20.0~300.0 5.0~40.0 [58 ]
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为: ...
High-temperature thermodynamics of aqueous alkali-metal salts
3
1992
... 对于水热条件下Na2 SO4 体系的密度实验数据文献已有全面而详实的报道,超过1 000个实验数据跨越温度0~300.0 o C,压力0.1~80.0 MPa(表1 ).关于K2 SO4 体系的密度数据相对较少(表2 ).Obšil等[48 ] 和Azizov[58 ] 实验研究了20.0~300.0 o C,10.0~30.0 MPa条件下K2 SO4 溶液的密度;Ellis[53 ] 和Saluja等[54 ] 研究获得了0~200.0 o C,0.1~2.0 MPa条件下K2 SO4 溶液的密度.不同来源的K2 SO4 体系密度数据之间具有较好的一致性,其密度数据的跨度范围为0~300.0 o C,0.1~40.0 MPa. ...
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
... Summary of density data of K
2 SO
4 -H
2 O system
Table 2 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 0~90.0 0.1 [57 ] 20.0~300.0 5.0~40.0 [58 ]
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为: ...
Volumetric properties of Na2 SO4 -H2 O and Na2 SO4 -NaCl-H2 O solutions to 523.15 K, 70 MPa
1
2015
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
The bulk properties of the Na2 SO4 -H2 O system in a wide range of the parameters of state
1
2000
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
A model for calculating the heat capacity of aqueous solutions, with updated density and viscosity Data
2
2009
... Summary of density data of Na
2 SO
4 -H
2 O system
Table 1 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 25.0~200.0 1.1~68.6 [49 ] 5.0~60.0 0.1 [50 ] 20.0~200.0 2.0~10.0 [51 ] 0~50.0 0.1~80.0 [52 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 25.0~250.0 0.1~40 [55 ] 30.0~300.0 2.4~39.8 [56 ] 5.0~100.0 0.1 [57 ]
表2 K2 SO4 -H2 O 体系密度的实验数据 ...
... Summary of density data of K
2 SO
4 -H
2 O system
Table 2 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 0~90.0 0.1 [57 ] 20.0~300.0 5.0~40.0 [58 ]
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为: ...
The density and partial properties of K2 SO4 -H2 O solutions from room temperature to 573 K
2
1998
... 对于水热条件下Na2 SO4 体系的密度实验数据文献已有全面而详实的报道,超过1 000个实验数据跨越温度0~300.0 o C,压力0.1~80.0 MPa(表1 ).关于K2 SO4 体系的密度数据相对较少(表2 ).Obšil等[48 ] 和Azizov[58 ] 实验研究了20.0~300.0 o C,10.0~30.0 MPa条件下K2 SO4 溶液的密度;Ellis[53 ] 和Saluja等[54 ] 研究获得了0~200.0 o C,0.1~2.0 MPa条件下K2 SO4 溶液的密度.不同来源的K2 SO4 体系密度数据之间具有较好的一致性,其密度数据的跨度范围为0~300.0 o C,0.1~40.0 MPa. ...
... Summary of density data of K
2 SO
4 -H
2 O system
Table 2 温度范围/o C 压力范围/MPa 参考文献 25.0~300.0 9.0~30.0 [48 ] 50.0~200.0 2.0 [53 ] 25.0~100.0 0.6 [54 ] 0~90.0 0.1 [57 ] 20.0~300.0 5.0~40.0 [58 ]
对于Na2 SO4 和K2 SO4 体系,公式(9)和公式(14)转化为: ...
Vapor pressures and partial molal volumes of aqueous solutions of the alkali sulfates at 25°
1
1937
... 依据公式(16)和表3 中的参数,我们计算不同温度压力条件下Na2 SO4 和K2 SO4 的标准偏摩尔体积.计算结果显示,25 ℃ 2 SO4 和K2 SO4 的标准偏摩尔体积分别为12.26和33.42 cm3 /mol,与实验测得的Na2 SO4 (12.09 cm3 /mol)和K2 SO4 (33.70 cm3 /mol)[59 ] 的结果吻合得很好.与此同时,我们还计算了Na2 SO4 和K2 SO4 的标准偏摩尔体积随温度压力的变化(图3 ).从图3 可看出,Na2 SO4 和K2 SO4 的标准偏摩尔体积随温度强烈的变化,在整个温度范围内,随温度的升高而降低,随压力的增大而增大. ...
Investigation of the precipitation of Na2 SO4 in supercritical water
3
2017
... 获得了压力对Na2 SO4 和K2 SO4 的标准摩尔体积和活度系数的影响之后,依据公式(4)和公式(5)计算了水热体系中Na2 SO4 和K2 SO4 的溶度积和溶解度.从表4 可以看出,模型计算的结果和实验测得的溶解度之间吻合较好.对于Na2 SO4 体系,Voisin等[60 ] 研究了压力为25 MPa时,水热体系中Na2 SO4 溶解度随温度的变化,并给出了计算Na2 SO4 溶解度的经验公式.从表4 我们发现,本文模型计算的Na2 SO4 溶解度与Voisin等[60 ] 经验公式计算的结果吻合较好.但是对于K2 SO4 体系,高压的实验数据基本都是在超临界条件以上,只能和饱和蒸汽压的实验室数据对比. ...
... [60 ]经验公式计算的结果吻合较好.但是对于K2 SO4 体系,高压的实验数据基本都是在超临界条件以上,只能和饱和蒸汽压的实验室数据对比. ...
... P = 25 MPa
50.0 3.53 3.19 [60 ] 100.0 2.34 2.74 150.0 2.33 2.53 180.0 2.39 2.48 200.0 2.41 2.46 220.0 2.37 2.40 图5 和图6 分别表示水热体系模型计算的Na2 SO4 和K2 SO4 的溶度积的自然对数和溶解度.从图5 a中可以看出,压力能够促进电解质溶解平衡常数的增加,在150 o C,压力为饱和蒸气压,10 MPa和30 MPa时,Na2 SO4 溶解平衡常数的自然对数分别为-2.71,-2.65和-2.55;相同条件下K2 SO4 溶度积的自然对数分别为-3.50,-3.45和-3.36(图6 a).但是压力对电解质溶解度的影响,却恰好与之相反.从图5 b中可以看出,随着压力的增大,电解质的溶解度却迅速降低.150 o C时,随着压力的增加,Na2 SO4 的溶解度从饱和蒸气压的2.89 mol/kg降低到10 MPa的2.73 mol/kg,最后降低到30 MPa的2.48 mol/kg.在相同温压条件下,K2 SO4 的溶解度分别为1.57,1.26和1.00 mol/kg.随着温度的升高,溶解度的减少量更大(图6 b).这是因为压力对Na2 SO4 和K2 SO4 的溶度积和平均活度系数都有正向的促进作用,但是压力对Na2 SO4 和K2 SO4 平均活度系数的影响更明显,结果就导致了Na2 SO4 和K2 SO4 溶解度的降低. ...






 甘公网安备62010202000687
甘公网安备62010202000687