作者简介:杜佳媛(1993-),女,黑龙江哈尔滨人,硕士研究生,主要从事水资源利用与水污染控制研究.E-mail:dujiayuan@tju.edu.cn
氧化石墨烯具有相对较大的表面积,其表面存在的丰富的含氧官能团使其具有良好的亲水性,能稳定地分散在水溶液中,是一种潜在的优质吸附剂。现有研究表明,氧化石墨烯对重金属及阳离子染料废水的处理效果明显优于广泛应用的传统吸附剂,然而由于其自身结构缺陷的原因,其应用还受到很多局限。介绍了氧化石墨烯的制备和结构,并重点针对氧化石墨烯及其复合材料对重金属离子和有机污染物的吸附行为、吸附机理、吸附模型及吸附影响条件等进行了综述,最后对氧化石墨烯及其复合材料在吸附方向的研究和应用前景进行了展望。
First author:Du Jiayuan(1993-), female,Haerbin City, Heilongjiang Province, Master student. Research areas include utilization of water resources and control of water pollution.E-mail:dujiayuan@tju.edu.cn
Graphene oxide, as an emerging material for contaminants removal,possesses relatively large specific surface area, and it shows good dispersion in water phase due to the hydrophilicessence resulted from abundant oxygen-containing functional groups on the edge, thus leading to a potential excellent adsorbent. Current studies revealthat, because graphene oxide is negatively-charged in a wide range of pHs, the removal efficiency of heavy metals and cationic dyes by graphene oxide is significantly higher than by traditional adsorbents, like activated carbon. However, its applications are still limited due to its structural defects. For example, its π domain is destructed during fabrication process. Therefore, certain structural modifications need to be conducted on the purpose of improving its performance, achieving a better result in water purification. This paper presented the preparation and structure of graphene oxide, and reviewed the adsorption behaviors, adsorption mechanisms, adsorption models and influence factors of heavy metals and organic pollutants on graphene oxide and its composites, respectively. In view of unresolved issues, further research should focus on comprehensive adsorption mechanisms, more facile and effectivemethods for structural modifications and the treatment of graphene oxide after adsorption process.
石墨烯自从2004年被英国曼彻斯特大学Geim项目组利用微机械剥离法成功制备之后[1], 由于其独特的结构、良好的机械强度、优异的导电性、独特的光学性能以及催化性质, 受到了学术界的广泛关注[2, 3], 对石墨烯衍生物的研究也随之成为热点。氧化石墨烯(Graphene Oxide, GO)是石墨烯的一种重要衍生物, 是利用超声、长时间搅拌或高速离心等方法, 将浓酸或强氧化剂氧化后的石墨剥离而成。
作为石墨化学氧化剥离的产物, GO的制备可以追溯到19世纪中期。传统的氧化石墨的制备方法有Brodie法[4]、Staudenmaier法[5]、Hummers法[6]。以上3种方法的基本原理相同, 均是石墨在强氧化剂以及强酸的作用下, 形成石墨层间化合物, 然后加入强氧化剂进行再次氧化, 最终得到氧化石墨。Brodie法及Staudenmaier法易产生有毒气体ClO2和I2等, 因此反应时间较短、环境污染程度较小的Hummers法便成为科研界制备氧化石墨的首选。此外, Hummers法制备的氧化石墨还具有含氧量较高、含氧官能团丰富、碳层破坏程度低等优点。目前, 在Hummers法的基础上, 科研工作者们不断探索改进, 通过改变氧化剂用量或者增加预氧化等方式以期获得低晶格缺陷的氧化石墨, 制备的氧化石墨经超声或者剧烈搅拌后即可层层剥离获得GO。改良Hummers法已成为实验室小型制备GO的首选方法。
在环境治理领域, 石墨烯及其衍生物广泛应用于新型吸附剂和光催化材料的制备、新生代水处理膜的合成及监测环境污染的电极材料等方面。本文将重点论述GO及新型GO复合材料作为吸附剂对水体中重金属及有机污染物的吸附过程及吸附机理。
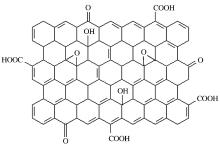
GO的本质是单层氧化石墨, 其理论表面积高达2 620 m2/g[7]。现有研究表明, GO由2种区域构成, 包括未被氧化的芳香区域(sp2碳原子)及由于氧化破坏而形成的脂肪六元环区(sp3碳原子), 两者的相对大小与氧化程度有关[8]。关于GO的具体结构, 目前有Hofmann模型、Ruess模型、Scholz-Boehm模型、Nakajima-Matsno模型、Lerf-Klinowski模型等[9]。然而由于表面含氧官能团性质和分布的不确定性, 以及缺少足够精确的分析手段, 所以GO精确的结构仍然有待确定[9]。关于GO官能团的分布, 目前被广泛接受的是Lerf-Klinowski模型(其结构示意图如图1所示), GO基面以石墨烯结构为主并带有一定量的环氧基及羟基, 单片边缘处分布着羧基及羰基[8, 10~12], 从而使其具有独特的边缘亲水、中央疏水的二维两性结构[13]。GO曾一直被视为一种亲水物质, 但最近的研究发现, 其从边缘到中央, 又有从亲水到疏水的变化趋势, 表现为两亲性[14~16]。亲水性是GO在水中可以稳定分散的一个主要因素, 同时也有学者认为表面的静电力排斥也是使其可以在水中稳定分散的因素。这些表面含氧官能团的引入使得石墨的层间距从0.34 nm增加到0.65 nm, 并且由于氧化石墨的亲水性, 水可以进入层间结构, 进一步将层间距由0.65 nm增加到1.15 nm, 层间距的增加使其剥离起来更容易, 这使得GO的制备成本会低[17]。同时, 含氧官能团的存在使得GO在水溶液中具有良好的分散性, 有利于衍生物的制备, 并且这些官能团可以作为化学修饰或者功能化的活性位点, 进一步引进新的基团, 使聚合物与亲水性的分子可以通过层间氢键、离子键、共价键等作用插到GO层间, 赋予GO良好的兼容性。
虽然含氧官能团的引入赋予了GO很多新的不同于石墨烯的性质, 但与此同时, 石墨烯的完美大π 结构遭到破坏, 其导电性下降。含氧官能团的存在可造成GO以下几个方面的性质缺陷:①GO虽然可以在水中稳定存在, 但其在盐溶液及其他生物溶液中会发生团聚[18]; ②吸附后, 作为吸附剂的GO由于自身良好的亲水性而难以从水中分离[19, 20]; ③由于其自身的大π 结构在制备时遭到破坏, 难以与芳香族有机物相互作用, 并且结构不稳定。因此, 针对以上结构缺陷, 可以从以下2个方面对GO的表面积、疏水性、电导率、储存模量等性质进行优化。
首先, 可以对GO进行表面化学修饰, 引入可以与水体中污染物相互作用或者改变GO自身亲疏水性等性质的官能团[21, 22]。一方面, 能够提高官能团的数量、种类及活性位点的吸附效率; 另一方面, 可以降低氢键施主基团的密度, 削弱层间氢键强度, 从而降低GO的亲水性, 使其易溶解于有机溶剂[23]。目前, 已报道的用于GO表面化学修饰的物质有阳离子表面活性剂[24]、EDTA[25]、乙二胺三乙酸[26]、长链脂肪族胺[27]、甲硅烷[28]等。Shen等[29]在GO的基础上, 采用预还原— 磺化— 再还原的方法成功制备了表面带有磺酸基团(-S
另外, GO复合材料的制备也是近期研究的热点之一。表面大量含氧官能团的存在, 使得GO碳层间带负电, 带正电的阳离子可以进入层间, 从而增大层间距, 为其他聚合物及无机纳米颗粒的进入提供便利条件[33]。目前, 对于GO与金属纳米颗粒和聚合物的复合材料研究的较多。金属/GO复合材料的优势在于有效增加了GO的比表面积, 同时有利于电子迁移, 解决了GO由于结构受到破坏所导致的电导率差的问题。制备方法主要有溶液共混法、熔融共混法及原位聚合法等, 其中溶液共混法应用最多。目前已成功与GO复合的金属纳米颗粒主要有Cu[34], Ag[35], Au[36], Pt[37], Mg[38]和Zn[39]等, 但这些制备方法仍不成熟, 有很大的改进空间。GO与不同的金属结合方式不同, 这取决于金属本身的性质。Li等[39]研究表明, Zn纳米颗粒可以通过静电作用与带负电的含氧官能团中的氧原子相互作用, 或者通过分子间的氢键作用和配位键与GO表面的官能团结合。Park等[38]对碱土金属纳米颗粒与GO的复合进行了研究, 一方面碱土金属纳米颗粒可以紧紧地绑定在GO边缘的羧基官能团上; 另一方面, 环氧基团开环形成-OH, 可以和羰基一起与碱土金属纳米颗粒相互作用。GO与聚合物的复合很好地利用了聚合物的特性, 弥补了GO材料自身导电性不良、结构不稳定的缺点, 使其具有更出色的导电性能、稳定性能及力学性能等[40]。此外, 也有一些关于GO与有机分子复合材料的研究报道。纳米材料与有机分子的复合材料可以有效增加储存模量、自身硬度、电导率及热稳定性[41, 42]。Zhang等[43]和Zhu等[44]等分别利用π -π 作用和酰胺化反应将酞菁类分子修饰到GO表面。Li等[42]通过模板牺牲法将聚吡咯与GO复合。Bao等[41]采用原位热聚合的方法将六氯环三磷腈和缩水甘油修饰的GO与环氧树脂合成纳米复合材料。Wang等[45]在GO的基础上, 通过溶剂热法将GO分别与烯丙胺及异氰酸苯酯复合, 前者增加了GO的亲水性, 后者增加了GO的疏水性, 为进一步合成复合材料打下基础。
目前还新兴了一种碳纳米管、石墨烯或者还原性氧化石墨烯与GO复合的全碳复合材料。以碳纳米管为例, 将碳纳米管加入GO溶液中, 碳纳米管会嵌入相邻的GO层, 从而阻止了GO层间的堆积。一方面增加了其比表面积, 另一方面碳纳米管作为额外的传电通道, 增加了整体的导电性[16]。
来自于矿业、工业垃圾、管道腐蚀等的重金属污染物已经成为水栖环境及饮用水源地最普遍的污染物之一。处理水体中重金属污染物的传统方法有3种:一是利用中和沉淀法、硫化物沉淀法等化学反应的方法将重金属离子从水体中以沉淀的形式回收; 二是利用离子交换、萃取法、吸附等物理化学的方法将水体中重金属离子回收; 三是利用水中微生物或者植物体对重金属离子的吸收、积累、富集等生物处理法将其回收[46]。与其他方法相比, 吸附法简单易行、操作简便、避免二次污染, 是处理水体中重金属污染的常用方法。而吸附剂的选择决定了处理效果, 是水处理中的关键因素。
GO合成条件相对温和, 且原料相对廉价易得。与其他的新型吸附剂相比, GO表面积大, 成本较低, 其表面羧基及羟基的存在使其具有良好的亲水性, 可以与金属离子发生静电作用、离子交换等相互作用, 表面上的氧原子具有孤对电子, 可以通过共用电子对与金属离子发生络合作用, 使得水体中的金属离子被吸附在上面。现有研究表明, GO对金属离子的吸附能力显著优于传统吸附材料活性炭[30], 及目前有报道的纳米碳管(Carbon Nanotube, CNT)等吸附材料[47]。
GO对污染物的作用主要依赖于其自身结构中的含氧官能团及芳烷基两部分。含氧官能团主要倾向于与亲水性物质相互作用, 而芳烷基中的π -π 共轭结构更倾向于和疏水性物质相互作用。GO与环境污染物的作用机理主要有静电作用(Electrostatic Interaction)、π -π 交互作用(π -π Bonding)、氢键作用(Hydrogen Bonding)、路易斯酸碱作用(Lewis Acid-base Interaction)和络合作用(Complexation)等5种。
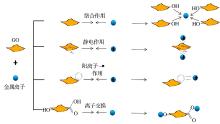
GO对重金属离子的吸附主要取决于其表面含氧官能团的种类和数量以及基面上的π -π 共轭结构, 而吸附作用力来源于静电引力、络合作用和阳离子-π 键作用等[48]。在酸性条件下, 刚开始随着pH 增大, GO电离程度增加, GO表面带负电荷量增加, 静电作用是GO吸附重金属的主要的作用机理。当pH逐渐增大, 此时GO表面的羟基可以与金属离子形成配位键, 生成络合物, 络合作用及金属离子交换开始成为GO与重金属作用的主要机理[49~51]。GO与金属离子作用机理如图2所示。
吸附等温曲线是指在一定温度下溶质分子在两相界面上进行的吸附过程达到平衡时它们在两相中浓度之间的关系曲线[52]。GO对于环境污染物的最大吸附能力通常通过吸附模型(表1)得到, 其对于重金属的吸附量一般采取Langmuir吸附曲线拟合得到。
| 表1 GO在水相中常用的吸附等温线模型 Table 1 Isotherm fitting models for GO in the aqueousphase |
pH、离子强度、温度、GO含氧官能团的数量和种类、自然环境中天然有机质(Natural Organic Matter, NOM)的存在等诸多因素, 都会影响GO对重金属离子的吸附[59~61]。溶液pH对吸附的影响主要体现在pH会影响吸附质和吸附剂所带电荷, 从而影响二者的静电作用[62, 63]。一方面, pH会影响GO表面的带电性质。GO在水相中的吸附性能取决于等电点, 若溶液的pH高于等电点, GO表面由于羧基及羟基的存在带有负电荷, 此时对溶液中带正电荷的重金属离子吸附能力较强。相反, 若溶液的pH低于等电点, 此时GO表面带有正电荷, 吸附效果相应削弱。另一方面, pH亦会影响吸附质的带电性质。在不同pH下, 金属离子会形成不同的化合物, 此化合物的电性亦会影响GO对金属离子的吸附。总的来说, GO对于重金属离子的吸附适合在酸性条件下进行, 但若pH过小, 可能会使GO的结构破坏, 或者水体中的H+与重金属离子产生竞争吸附, 造成吸附能力下降。表2是GO对部分重金属吸附量最大时所对应的最佳pH, 由表2可知, 最佳pH一般都是在弱酸性条件下。所以在实际应用中, 最佳吸附pH需要靠实验进行确定, 并不是pH越低, 吸附能力越强。
离子强度主要通过以下3种作用影响GO对重金属离子的吸附:①离子强度会影响GO与吸附质之间的静电作用。静电作用是GO在低pH时吸附重金属的主要机理。当溶液中的离子强度增加时, 吸附质与吸附剂之间的静电作用便会相应减弱[67, 68]。与此同时, 疏水作用减弱, 但离子强度对络合作用的影响不大。②离子竞争吸附。加入溶液的电解质离子会与溶液本身的吸附质离子发生竞争吸附, 从而抑制重金属的吸附。随着离子强度增加, 吸附量下降[69, 70]。③与吸附质产生盐析或盐溶效应[71]。当电解质加入溶液后, 该离子会与溶液本身的吸附质离子争夺溶剂分子, 溶剂分子会向强电解质方移动, 造成弱电解质方的水合度及溶解度下降, 从而更倾向于被吸附[71]。GO对金属离子的吸附是吸热反应。在其他条件相同的情况下, 吸附量随着温度的升高而加大, 然而温度对吸附量的影响程度不大。
| 表2 GO及GO复合物对常见金属的吸附 Table 2 Existing data on adsorption of metal ion by GO and GO composites |
除此之外, NOM也是重要的影响因素。NOM主要包括腐殖质、多糖等[10, 72, 73]。NOM的主要官能团包括-COOH, -OH, -NH2等。GO对污染物的吸附大多是在自然环境中进行的, 此时便不可避免地受到NOM的影响[74, 75]。一方面, GO的稳定性会因为NOM的存在, 产生空间斥能而显著提高, 从而暴露出更多的吸附位点, 提高对重金属离子的吸附量[10]; 另一方面, NOM分子会通过氢键、路易斯酸碱作用或π -π 相互作用被GO吸附, 从而占据其表面官能团, 使其对重金属等的吸附量下降[76]。
GO由于其较强的机械强度, 较高的理论比表面积及较低的成本, 常被用来作为复合物的基体。Mi等[51]通过单向冷冻干燥法(UFDM)制备的GO气凝胶, 其独特的单向多孔结构对于Cu(Ⅱ )有很好的吸附效果, 其吸附速率远远高于CNT及活性炭对Cu(Ⅱ )的吸附速率, 在15分钟就可以达到吸附平衡。Chandra等[77]利用FeCl2和FeCl3制备出磁铁矿— 还原GO, 等同于将GO修饰到了磁性纳米材料上, 可以在吸附完成后利用磁力将GO分离出来, 比传统的高速离心法方便许多。树枝状聚酰胺(PAMAMs)末端存在着大量高活性官能团[78, 79], Zhang等[80]将PAMAMs与GO利用主链接枝法复合, 制备出PAMAM/GO复合材料, 其对Pb(Ⅱ ), Cd(Ⅱ ), Cu(Ⅱ )和Mn(Ⅱ )的吸附能力分别达到了568.18, 253.81, 68.68和18.29 mg/g。有研究将GO与光催化材料TiO2复合[81~83], 其在增加了电子传输能力的同时, 又增大了吸附能力。Lee等[81]在GO的异丙醇胶体溶液中, 搅拌加入二氧化钛前体, 制备出GO-TiO2, 此复合材料对Zn(Ⅱ ), Pb(Ⅱ )和Cd(Ⅱ )均有很好的吸附能力。Sun等[84]在碱性条件下, 利用ClCH2COONa还原GO, 制备出HOOC-GOs, 其显著增加了材料的比表面积和羧基官能团的数量, 有效增加了其作用位点。Jiang等[82]在200 ℃煅烧条件下, 采用液相沉积法制备了GO-TiO2复合材料, 此二维材料拥有巨大的比表面积及良好的电子传递能力, 大大提高了对于Cr(VI)的光解去除率。表2总结了GO复合物对常见金属离子的吸附量。Tan等[85]利用GO及聚乙烯醇(PVA)在真空条件下合成复合膜, 该材料对于Cu(Ⅱ ), Cd(Ⅱ )和Ni(Ⅱ )的吸附能力分别为72.6, 83.8和62.3 mg/g, 在10分钟之内基本可以快速达到吸附平衡, 并且该复合膜可以多次重复使用。
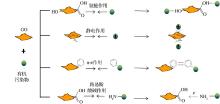
已有研究表明, GO对有机污染物的吸附主要发生在GO的未氧化区域[86], 且吸附能力随着GO氧化程度的升高而下降[87]。GO在制备的过程中, 石墨基面上的sp2碳π -π 共轭结构遭到破坏, 并且引入了氧化基团, 使其疏水性下降。因此, 相比于石墨烯凭借自身的大π 结构可以通过疏水作用及π -π 交互作用与非极性芳香族化合物(菲、萘、芘、多氯联苯等)[55, 88, 89]作用, GO则更倾向于与阳离子型有机物和极性芳香烃类化合物作用。水体中可以与GO作用的有机污染物大致有阳离子染料、极性芳香族化合物(蒽醇、萘酚、双酚A、1-萘胺)[87, 89~91]及高分子有机物(有机酸、DNA、蛋白质等)[92~94]等。但由于π 结构的缺陷和表面官能团的存在, GO对有机物的吸附能力有限。根据研究, GO对阳离子染料有很高的吸附量, 其吸附机理主要包括氢键、静电作用及π -π 作用[95, 96], 但是GO对阴离子染料的吸附能力很弱。GO对极性芳香族有机物的吸附主要是依靠氢键作用、静电引力、π -π 作用及路易斯酸碱作用[87, 97, 98]。GO主要通过π -π 作用与高分子有机物相互作用[92~94]。除此之外, 虽然没有明确的报道表明共价键的存在, 但是其可能发生在含-OH, -COOH和-NH2等官能团的有机物与GO的相互作用中。GO与水体中有机污染物作用机理示意图如图3所示。表2中的吸附等温线均可用于拟合GO对有机物的吸附, 其中Langmuir及Freundlich是最常应用的吸附模型。
Gao等[54]研究了GO对四环抗菌素(Tetracycline antibiotics)的吸附。一方面, 四环抗菌素的氨基易于质子化, 可与GO形成阳离子-π 键; 另一方面, 四环抗菌素的苯环可与GO发生π -π 作用。这使得四环抗菌素在GO上的最大吸附量可达313 mg/g。吸附等温线符合Langmuir模型, 吸附量受溶液pH及离子强度的影响。Pei等[99]研究了GO对1, 2, 4-三氯苯、2, 4, 6-三氯苯酚等多种有机污染物的去除机理, 发现GO主要依靠羟基与有机物在溶液中形成氢键而将其去除。但是在水环境中, 氢键作用会相应减弱。Ramesha等[96]研究了GO对阳离子染料(罗丹明B、亚甲基蓝)的吸附, 发现其去除效果均优于石墨烯, 静电引力是主要的作用机理。有研究表明[95], 随着GO氧化程度的增加, 其自身的剥离程度及在水中的分散性也随之增加, 对亚甲基蓝的吸附量呈指数增加。
石墨烯(包括GO)复合材料是近年的研究热点[100, 101], GO由于其自身表面含氧官能团的存在, 而与很多聚合物的基体有很好的相容性, 并且可以利用其他材料的优势特质弥补GO本身由于制备时大π 结构遭到破坏的结构劣势。现在对复合材料的研究大多停留在力学性能、热学性能等表观性能的研究上, 对有机物的吸附并没有过多的深入研究[102, 103]。但是从现有的性能分析来看, 有很多新型复合材料在溶液中显示出了非极性的特性。
 | 图3 GO与有机污染物作用机理示意图Fig.3 Diagrammatic sketch for the mechanism of organic pollutant adsorption onto GO |
Yang等[104]成功制备了壳聚糖/GO复合材料(GO/GS), 普通的GO在使用后需要用高速离心去除, 成本过高, 而GO/GS复合材料可以采取低速离心或者过滤分离的方法, 易于去除。Wang等[105]利用氨水和GO磁搅拌加热后生成掺氮的GO, 其在制备过程中促进了GO的剥离, 增加了有效表面积, 并且由于氮存在空闲电子对, 可以作为催化剂, 促进氧化剂释放出自由基, 原位降解已吸附的污染物, 释放出有效吸附位点, 可以进行下一步吸附。
另一方面, 现在国际上已经制备出三维GO并成功应用于吸附油类、染料等有机污染物[100, 106]。三维结构可以赋予GO新的性质:柔韧性、多孔性、高活性比表面积等, 并且对其进行改性或者制备复合材料可以有效地调节其亲/疏水性。Liu等[107]成功制备了三维形态的GO海绵, 该材料对甲基紫及亚甲基蓝的吸附量分别为467和397 mg/g, 明显优于二维GO通过静电作用对阳性染料的吸附能力。Sui等[108]利用GO与聚乙烯亚胺相互作用制备出三维多孔GO, 该三维材料富含大量氨基官能团和丰富的多孔结构, 对酸性染料有很强的吸附作用, 克服了传统GO只能吸附碱性染料的弊端。
除此之外, GO也被制备成膜应用于水处理中。Lee等[109]将GO膜应用在膜生物反应器(MBR)中, 将膜分离处理单元与生物处理相结合, 应用到污水处理中。有研究表明[110], 气体(H2, N2, He, Ar)及有机溶剂的蒸气对GO膜的渗透通量都很小, 而水蒸气几乎没有渗透阻力, 其在通过氧化区域时可以与环氧基团及羧基形成氢键, 增加流动阻力, 在通过芳香基团部分时则阻力很小[110]。现有研究表明, GO膜可以应用在水体脱盐[111], 提纯分离(水、石油、化工)[112, 113]等方面。虽然性能优越的GO膜在水处理中可以得到广泛应用, 但其强度、应用稳定性仍需进一步提高, GO膜的分离机理及影响因素仍需进一步探究[114]。
首先, 目前对于GO的吸附机理研究不够深入。虽然GO的制备工艺已经相对成熟, 其在吸附方面的研究工作也已经做了很多。然而由于其自身结构仍有不确定性, 所以吸附机理及吸附模型仍需进一步探究。
其次, GO在污水处理方面想要取得突破性进展, 则需要在材料本身的制备入手。GO在处理含重金属及带有正电荷的染料废水中有很好的效果。但是在处理有机物废水时, 由于自身的结构被破坏而受到了限制。从目前的研究来看, GO材料若在水处理领域获得突破性进展, 则需要把研究重点放在环境、化学、材料交叉研究上, 如对GO自身结构的改性, 通过表面进行化学修饰引入新的官能团及GO的复合材料的研究制备, 从而可以弥补自身的结构缺陷, 广泛应用于各类废水的处理中。
最后, GO在自然水体中的应用、同时完成对含有机物及重金属离子废水的处理、吸附后, GO后置处理也应该同样受到关注。可以考虑深入研究将GO制备成三维材料或者放在其他材料载体上制备成力学性能好、易回收的吸附材料。GO及其复合材料在水处理应用中具有广泛前景, 但是由于受到价格等因素的限制, 大量、广泛地应用于水处理领域尚需时间。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|